How is SARS-CoV-2 Detected by a RT-PCR Test?
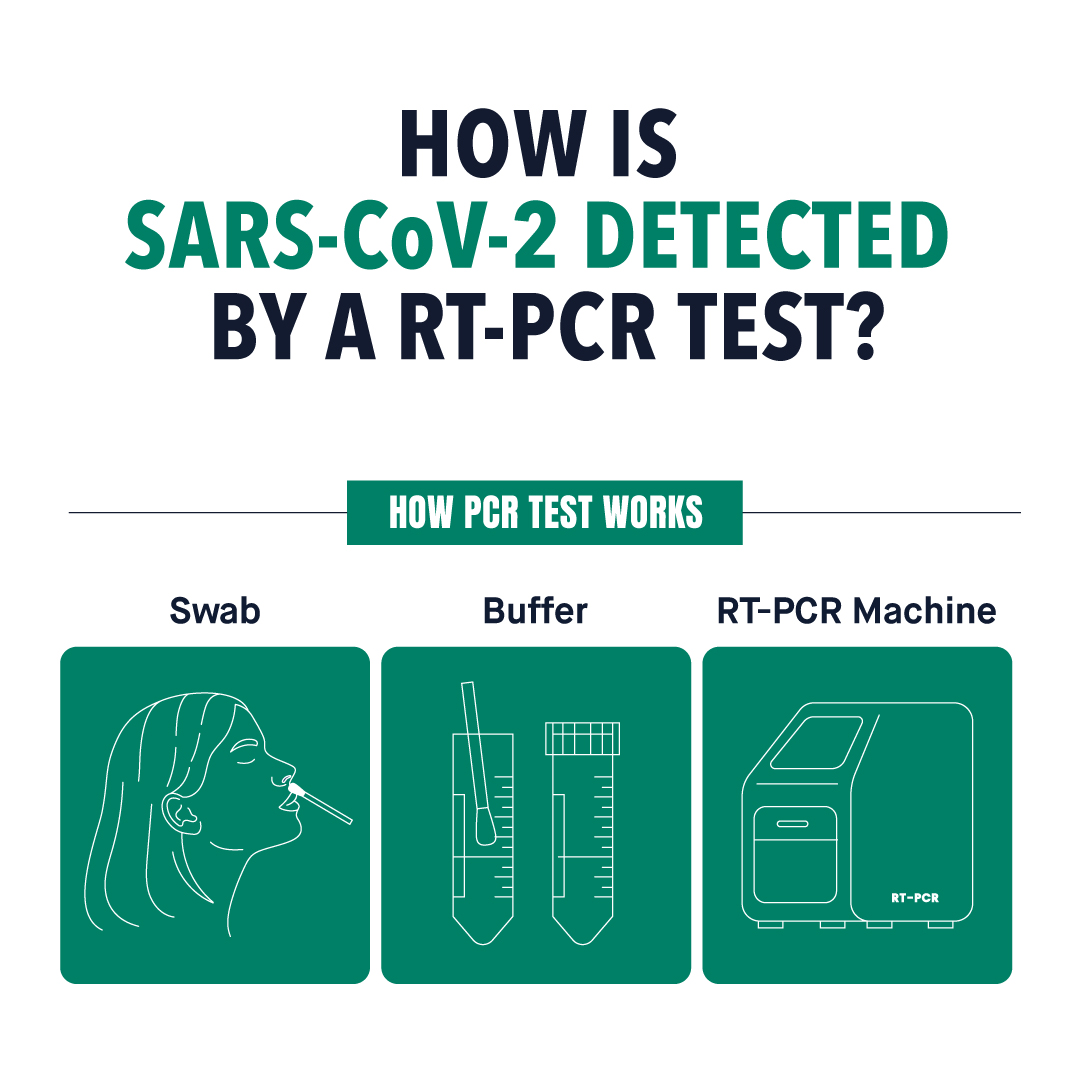
Despite the development of other methods of testing, polymerase chain reaction (PCR) testing remains the golden standard for the detection of SARS-CoV-2. Given the pivotal role of rapid and large-scale testing in transmission control and patient management, it is no surprise that efficient diagnostic devices are still in high demand. Out of the various diagnostic methods currently used for the diagnosis of infections with SARS-CoV-2, nucleic acid amplification tests (NAATs) are the primary methods of choice, with real-time RT-PCR remaining among the most reliable, accessible, and widely used methods. Read along to find out more about the use of RT-PCR method in the detection of SARS-CoV-2, its procedure, and the optimal timing for testing.
What is an RT-PCR Test?

Due to the large overlap between the two diagnostic methods, it could be beneficial to briefly introduce PCR testing before delving into RT-PCR testing. Polymerase Chain Reaction (PCR) testing is a diagnostic method which enables the detection of a specific sequence of viral RNA or DNA through multiple replications in a process known as amplification. Amplification allows for highly sensitive detection even in the cases where the viral load may be very low. However, for the detection of RNA viruses such as SARS-CoV-2, viral RNA must first be converted to deoxyribonucleic acid (DNA) through a process known as reverse transcription PCR (RT-PCR). Thus, the detection of SARS-CoV-2 with the RT-PCR method involves the conversion of a short piece of viral RNA from the collected samples into DNA, and its replication through many cycles until the presence of the virus is detectable. In real-time RT-PCR, the method also allows for the amplification and detection processes to proceed simultaneously, which slightly reduces turn-around time compared to regular PCR tests. You can find more detailed information on the required steps for the detection of SARS-CoV-2 by RT-PCR testing.
How does an RT-PCR Test Detect SARS-CoV-2?

The required steps for the detection of SARS-CoV-2 by RT-PCR testing include sample collection, transportation of the samples to the laboratory, specimen lysis, RNA purification via nucleic acid extraction, along with RT-PCR amplification, detection, and analysis. Due to the inclination of the virus to degrade quickly after sample collection, various transport media, such as our Viral Nucleic Acid Transport Medium and Viral Transport Medium, have been developed for the safe and efficient transportation and preservation of viruses for transfer to the laboratory, analysis, and further research.
Following the transportation of the samples to a laboratory, samples are lysed via physical, chemical, or enzymatic methods to release SARS-CoV-2 RNA from host cells and virions. Then, nucleic acids are extracted and purified to remove contaminants, cellular debris, and other potential inhibitors which could potentially interfere with the rest of the procedure. Extraction and purification of the viral RNA is critical for the achievement of optimal sensitivity and can be performed by manual methods or through the use of kits, such as our Viral Nucleic Acid Purification Kit.
Next, an enzyme called reverse transcriptase produces a cDNA from the SARS-CoV-2 RNA, which functions as a template throughout the PCR amplification cycles. The PCR amplification cycle is composed of three main steps, which are denaturation, annealing, and extension. The presence of the virus is typically detectable following 30 to 40 cycles of amplification. In real-time RT-PCR testing, there is a fluorescence signal enabling simultaneous detection during the amplification process, and the PCR cycle at which the fluorescence signal crosses the threshold for positivity is named the threshold cycle (CT).
How does an RT-PCR Test Differ from a Rapid Antigen Test?
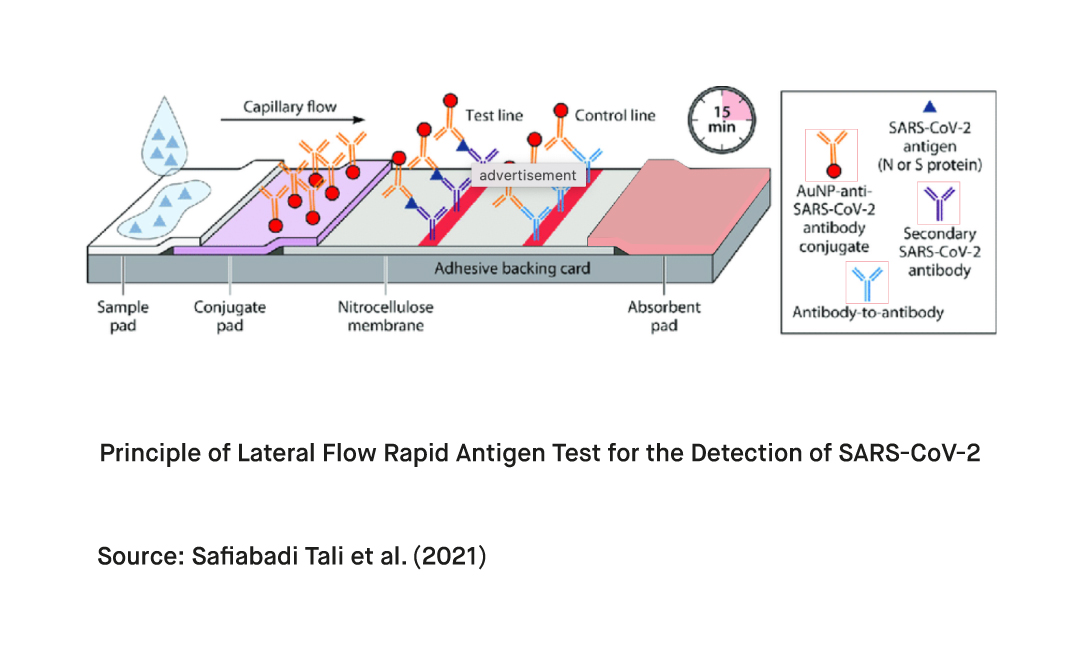
Unlike RT-PCR tests and other types of nucleic acid amplification tests (NAATs), rapid antigen tests, such as our RapidFor™ SARS-CoV-2 Rapid Antigen Test Kits, do not depend on the detection of viral RNA. Although both methods check for active infections with the virus, antigen tests rely on involves the use of a series of special antibodies to identify proteins specific to SARS-CoV-2 such as its nucleocapsid protein or spike protein.

Many rapid antigen tests designed for the detection of SARS-CoV-2 use a sandwich immunodetection method in lateral flow test format. Following the transfer of the sample to the assigned well on the test strip, the liquid travels along the surface of the kit through capillary flow. If the virus is present, the viral antigens in the sample bind to the line of labeled mobile antibodies on the strip. These migrating antigen-antibody complexes are captured by another line of immobilized antibodies, which causes the label of the captured antigen-antibody complexes to appear as a colored line on the test strip.
Antigen detection offers several unique benefits from a public health standpoint, including accessibility, convenience, affordability, and rapid results. Moreover, it allows for point-of-care testing and self-testing. However, given its excellent sensitivity, specificity, and overall accuracy, RT-PCR tests such as our SARS-CoV-2 Detection Real-Time PCR Kit, is widely considered as the golden standard in the detection of SARS-CoV-2.
When Should You Use an RT-PCR Test?

Given that different markers of SARS-CoV-2 appear, peak, and disappear at different periods following the onset of infection, timing of sample collection is among the most central constituents affecting the accuracy of the results and a decisive factor for the determination of the optimal diagnostic method for a given case. Compared with rapid antigen tests, molecular methods, such as RT-PCR testing can not only detect lower viral loads with higher sensitivity but also can detect an infection throughout a wider period of time. In fact, while antigen-based methods are more likely to test positive for around two weeks following the onset of the symptoms, molecular methods, such as RT-PCR testing, remain highly sensitive as soon as one week before symptom onset for around three weeks.
References
Safiabadi Tali, S. H., LeBlanc, J. J., Sadiq, Z., Oyewunmi, O. D., Camargo, C., Nikpour, B., Armanfard, N., Sagan, S. M., & Jahanshahi-Anbuhi, S. (2021). Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clinical Microbiology Reviews, 34(3). https://doi.org/10.1128/cmr.00228-20
Synowiec, A., Szczepański, A., Barreto-Duran, E., Lie, L. K., & Pyrc, K. (2021b). Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): a Systemic Infection. Clinical Microbiology Reviews, 34(2). https://doi.org/10.1128/cmr.00133-20