Breast cancer is one of the leading causes of cancer-related morbidity and mortality among women worldwide. Early detection and vigilant monitoring are essential for managing the disease effectively and improving patient outcomes. Among several biomarkers utilized in clinical practice, Cancer Antigen 15-3 (CA 15-3) has emerged as a vital indicator for assessing disease progression, therapeutic efficacy, and potential recurrence. Vitrosens’ CA 15-3 Rapid Test Kit (FIA) provides healthcare professionals with fast, accurate, and reliable results, playing a pivotal role in enhancing breast cancer management and patient care.
Importance of Rapid CA 15-3 Measurement
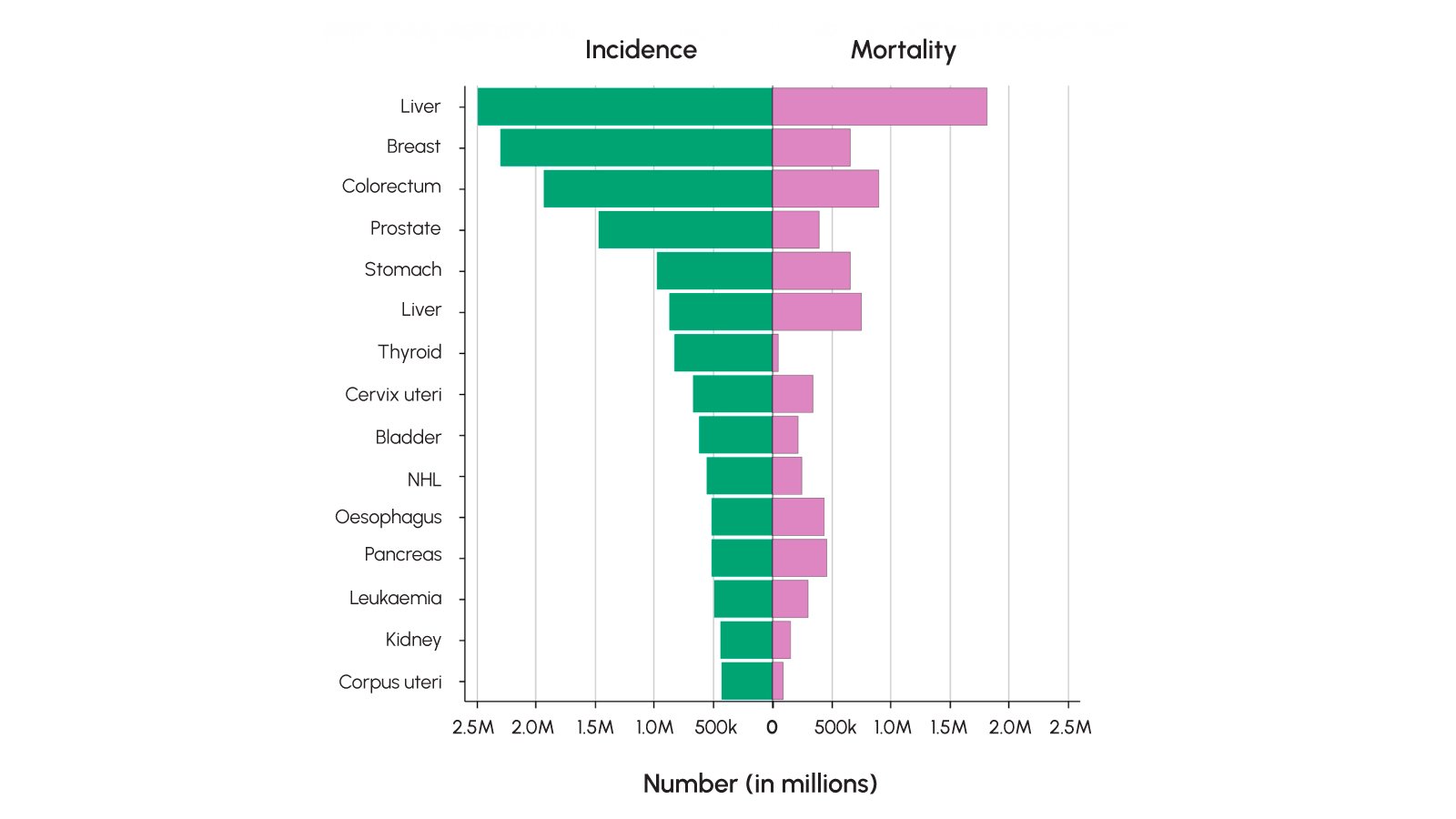
Breast cancer is the second most commonly diagnosed cancer and the fourth leading cause of cancer-related deaths worldwide. Although it is widespread, breast cancer is more treatable or manageable through early detection compared to some other types of cancer.
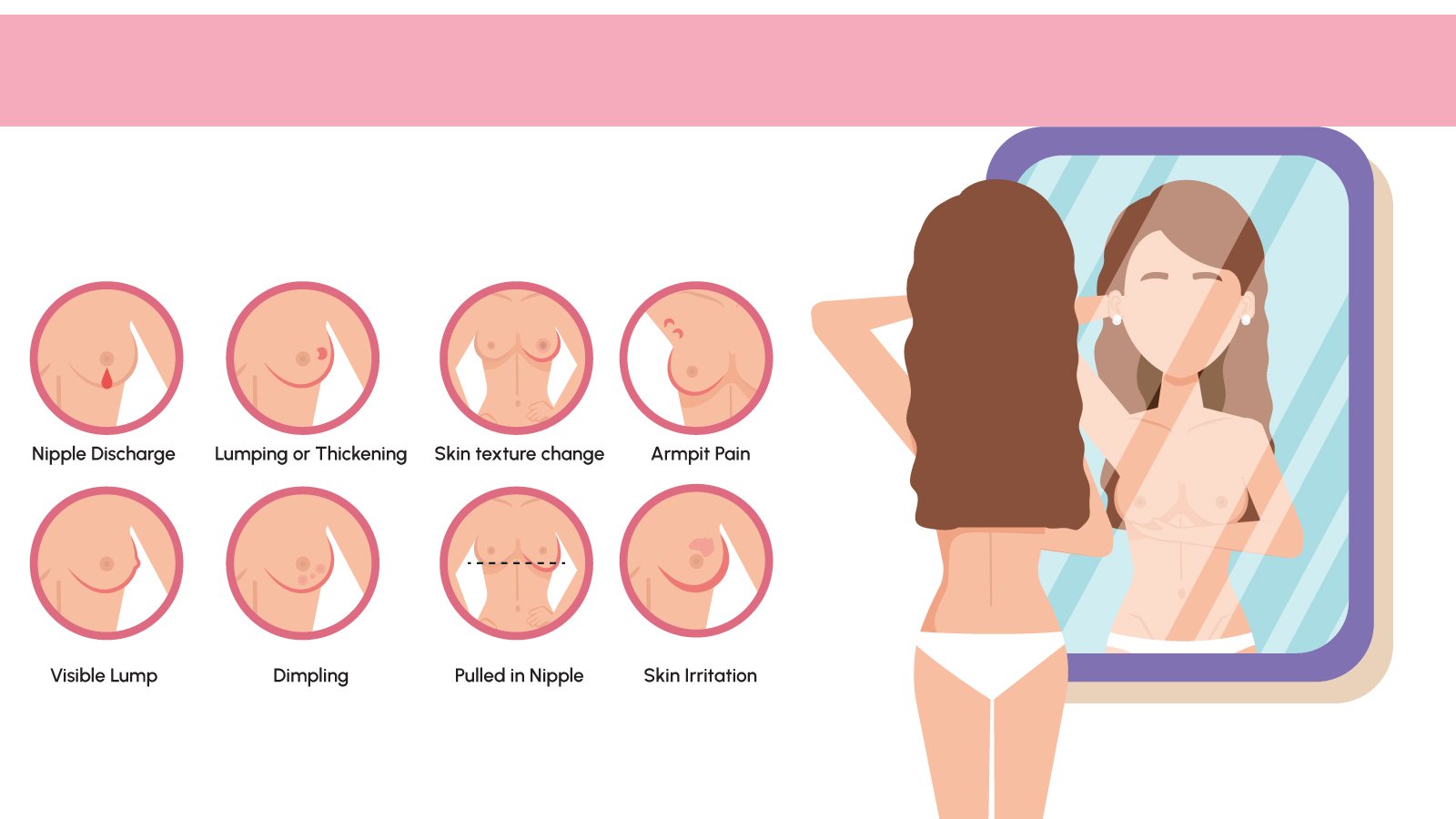
CA 15-3, a recognized tumor marker, helps in tracking breast cancer progression and evaluating treatment response. Elevated CA 15-3 levels often indicate cancer activity, though they can also occur with non-cancerous conditions. So symptoms indicating the necessity for monitoring include:
- Unexplained breast lumps
- Changes in breast shape or texture
- Persistent breast pain
- Unusual discharge from nipples
The CA 15-3 Rapid Test Kit (FIA) Technology
Vitrosens’ CA 15-3 Rapid Test Kit leverages Fluorescent Immunochromatography Assay (FIA), employing a double-antibody sandwich technique for highly sensitive and accurate antigen quantification.
Double-Antibody Sandwich Principle:
CA 15-3 antigen is captured between two specific antibodies, enhancing detection reliability.
Advantages of Fluorescent Immunoassay:
Delivers precise and consistent results, significantly reducing false positives and negatives compared to traditional methods.

Key Specifications of Vitrosens CA 15-3 Kit
Vitrosens’ CA 15-3 Rapid Test Kit provides clinical efficiency, accuracy, and ease of use. It uses serum or plasma samples, fully compatible with standard clinical practices. Key features include:
- Rapid Results: Obtain CA 15-3 quantification within 15 minutes.
- High Precision: Employing Fluorescent Immunoassay (FIA) for reliable quantitative analysis.
- Flexible Storage Conditions: Stable between 2°C to 30°C, removing cold-chain logistics constraints.
- Extended Shelf Life: 24-month shelf-life for improved inventory management and minimal waste.
Clinical Applications and Benefits
The CA 15-3 Rapid Test Kit significantly supports clinical decision-making in several scenarios:
- Monitoring Treatment Effectiveness:
Evaluates patient response to therapies, ensuring timely adjustments to treatments. - Detection of Recurrence:
Regular testing swiftly identifies potential cancer recurrence, facilitating early interventions. - Personalized Patient Management:
Enables tailored monitoring schedules and personalized patient care plans. - Emergency Clinical Decisions:
Rapid results allow immediate decision-making in urgent clinical situations involving breast cancer patients. - Enhanced Clinical Outcomes:
Timely and accurate monitoring promotes informed clinical decisions, directly improving patient prognosis.
Simple Testing Procedure
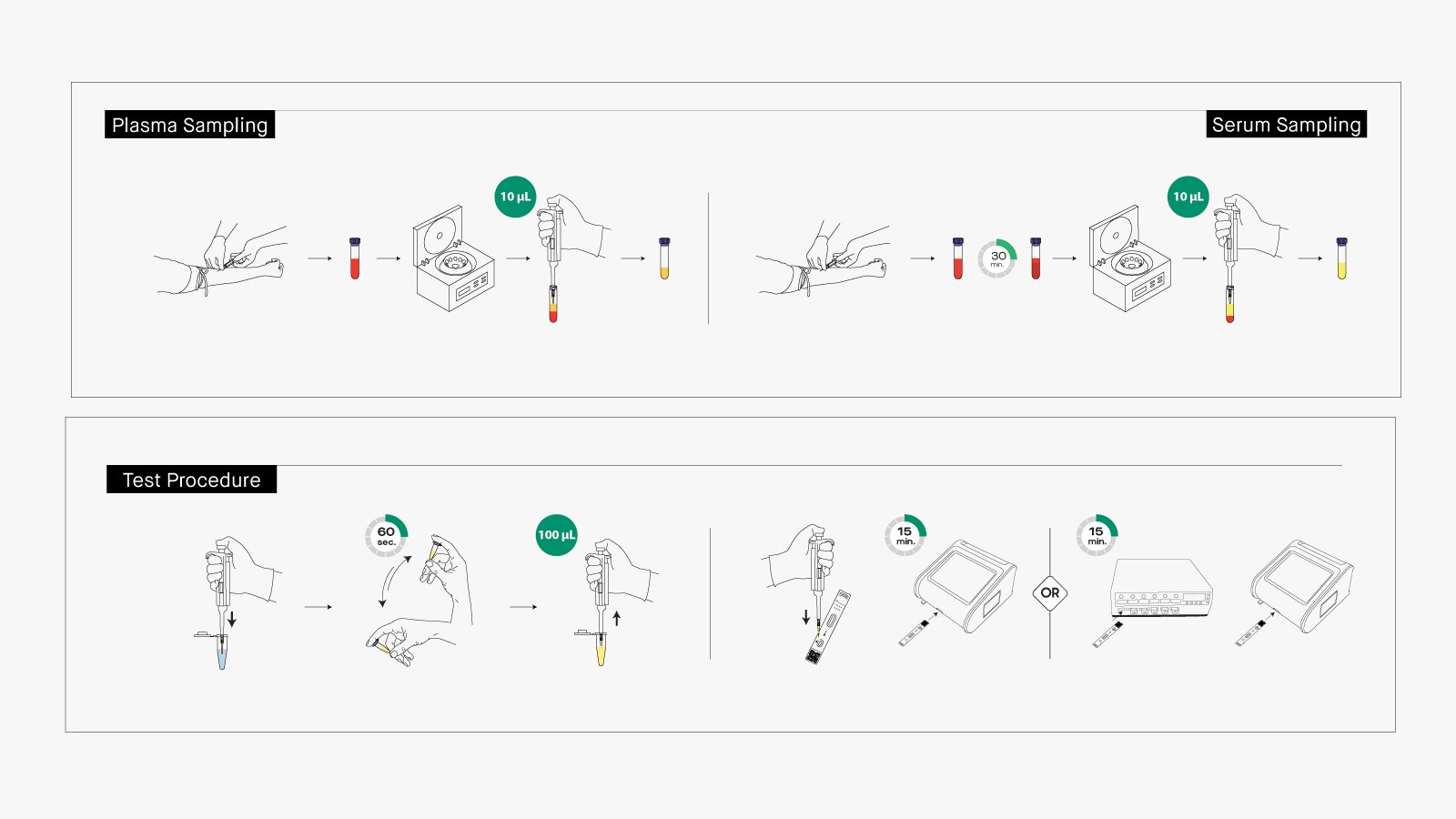
Vitrosens simplifies CA 15-3 measurement into straightforward steps:
- Collect blood via standard phlebotomy. Use EDTA tubes for plasma. Separate serum/plasma quickly to avoid hemolysis.
- Let reagents and samples reach room temperature.
- Turn on analyzer, select mode, scan reagent ID.
- Open test card pouch. Take 10μL sample, mix with diluent for 60 sec.
- Add 100μL mixture to test card and insert the test card into the instrument
- Quantitative CA 15-3 results available after 15 minutes, enabling immediate clinical action.
Conclusion
Roughly half of all breast cancers occur in women with no specific risk factors other than sex and age. Vitrosens’ CA 15-3 Rapid Test Kit (FIA) empowers healthcare professionals with rapid, precise antigen measurements, significantly enhancing breast cancer management. By reducing diagnostic delays and facilitating accurate, immediate clinical decisions, Vitrosens is revolutionizing patient care and disease monitoring worldwide.
Ready to improve breast cancer monitoring in your clinical practice?
Contact Vitrosens today for evaluation kits and comprehensive technical support!
Key References:
- WHO Breast Cancer Initiative: Global Strategy 2024.
- Van Eycken, Liesbet J., et al. (2025) “Future of population‐based cancer registries: A global perspective—A survey of population‐based cancer registries.”International Journal of Cancer.
- International Agency for Research on Cancer (IARC). (2024). Cancer Today – Data Visualization Tool. Global Cancer Observatory.
- Vitrosens Biotechnology. CA 15-3 Rapid Test Kit FIA – Technical Manual.


