As respiratory infections increase during peak seasons, clinical attention is often directed toward influenza, RSV, and SARS-CoV-2. However, human parainfluenza viruses continue to represent a substantial yet underrecognized burden in both pediatric and adult respiratory disease. Their overlapping symptom profiles with other respiratory pathogens make laboratory confirmation critical for precise diagnosis and appropriate clinical management.
Failing to detect HPIV can result in delayed diagnosis, unnecessary antibiotic prescriptions, and missed opportunities for infection control, particularly in high-risk settings.
Introduction: What Are Human Parainfluenza Viruses?
Human parainfluenza viruses are enveloped, single-stranded RNA viruses belonging to the Paramyxoviridae family. They are among the leading viral causes of acute respiratory tract infections worldwide, particularly in infants, young children, older adults, and immunocompromised patients.
Unlike influenza, HPIV infections lack specific antiviral treatment options. This makes early and accurate diagnosis essential to guide supportive care, avoid inappropriate therapies, and improve patient outcomes.
Understanding the Four Types of Human Parainfluenza Virus
HPIV Type 1
HPIV-1 is the most common cause of viral croup and is characterized by upper airway inflammation, barking cough, and inspiratory stridor. It typically circulates in biennial outbreaks during autumn and early winter, often affecting large pediatric populations.
HPIV Type 2
HPIV-2 also causes croup but is less frequently detected. Clinical presentation overlaps significantly with HPIV-1, making laboratory differentiation important in outbreak investigations and surveillance.
HPIV Type 3
HPIV-3 is primarily associated with lower respiratory tract infections such as bronchiolitis and viral pneumonia. It is a major cause of hospitalization in infants and can lead to severe disease in elderly and immunocompromised patients.
HPIV Type 4
HPIV-4 has historically been underdiagnosed due to limited routine testing. With the adoption of multiplex respiratory panels, HPIV-4 is increasingly recognized as a clinically relevant pathogen contributing to both mild and severe respiratory illness.
Clinical Overlap and Diagnostic Challenges
Parainfluenza virus infections often present with fever, cough, wheezing, sore throat, and respiratory distress. These symptoms are clinically indistinguishable from influenza, RSV, adenovirus, or SARS-CoV-2.
Without laboratory confirmation, clinicians may rely on empirical treatment strategies, which can lead to unnecessary antibiotic use and delayed isolation decisions. Rapid diagnostic testing plays a critical role in closing this diagnostic gap.
Why Detecting HPIV Is Especially Important in Winter Months
During winter respiratory seasons, multiple viruses circulate simultaneously, placing pressure on healthcare systems and primary care settings. In this context, identifying the causative pathogen quickly is essential for effective triage and patient flow.
HPIV detection during winter supports:
- Rapid differentiation of viral respiratory infections
- Reduced diagnostic uncertainty in pediatric cases
- Improved bed management in emergency departments
- More targeted use of additional diagnostic resources
The Role of HPIV Testing in Antibiotic Stewardship
Although HPIV infections are viral, they are frequently misinterpreted as bacterial respiratory infections due to overlapping clinical features. This misinterpretation contributes to unnecessary antibiotic prescribing.
Rapid identification of HPIV helps clinicians confidently withhold antibiotics when bacterial infection is unlikely. This supports antibiotic stewardship initiatives and aligns with global efforts to reduce antimicrobial resistance.
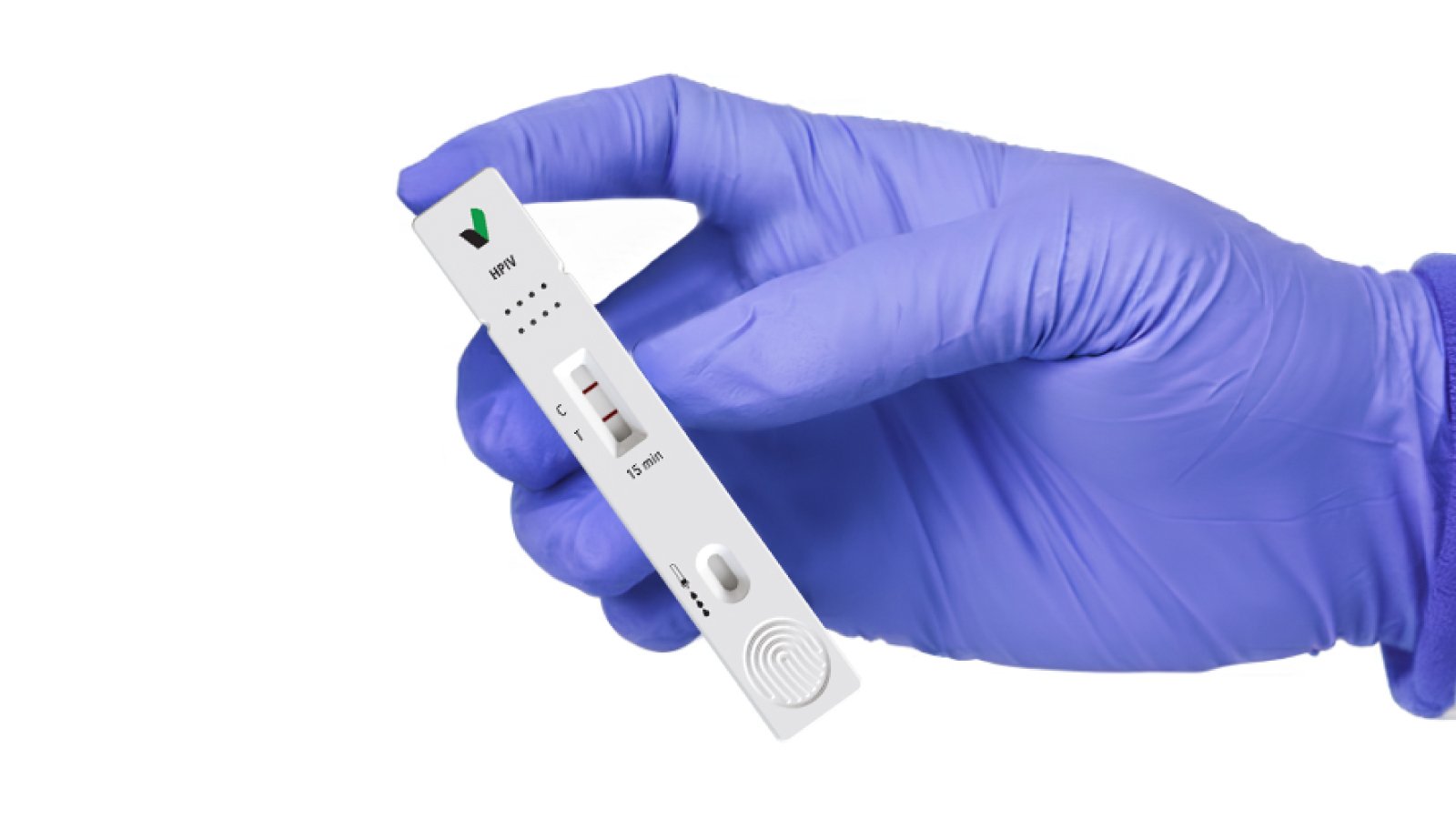
RapidFor™ HPIV Detection Solutions
RapidFor™ HPIV Rapid Test Kit
The RapidFor™ HPIV Rapid Test Kit is designed for the qualitative detection of human parainfluenza virus antigens directly from respiratory specimens. It enables fast, reliable identification of HPIV infections at the point of care.
Key clinical benefits include:
- Short turnaround time supporting immediate clinical decisions
- Simple workflow suitable for outpatient clinics and emergency settings
- Improved confidence in viral diagnosis during peak respiratory seasons
Multiplex Detection for Comprehensive Respiratory Diagnosis
RapidFor™ SARS-CoV-2 + FLU A/B + RSV + ADV + hMPV + HPIV Test Kit
In real-world clinical practice, respiratory symptoms are rarely caused by a single pathogen. Multiplex diagnostics provide a practical solution by detecting multiple viruses simultaneously from one sample.
The RapidFor™ SARS-CoV-2 + FLU A/B + RSV + ADV + hMPV + HPIV Test Kit includes parainfluenza virus detection as part of a comprehensive respiratory panel.
This multiplex solution enables:
- Faster differential diagnosis at first patient contact
- Reduction in repeat testing and sample recollection
- Improved workflow efficiency during respiratory surges

Visual overview demonstrating simultaneous detection of SARS-CoV-2, Influenza A/B, RSV, Adenovirus, hMPV, and HPIV.
Where HPIV Testing Fits in the Diagnostic Pathway
HPIV testing is particularly valuable in:
- Pediatric outpatient clinics
- Emergency departments
- Hospital admission triage
- Seasonal respiratory surveillance programs
By integrating HPIV detection into routine respiratory testing algorithms, healthcare providers gain a more complete picture of circulating pathogens and can tailor patient management accordingly.
Conclusion
Human parainfluenza viruses remain a significant but often underdiagnosed cause of respiratory illness across all age groups. Accurate and timely detection is essential for informed clinical decision-making, effective infection control, and responsible antibiotic use.
With both the RapidFor™ HPIV Rapid Test Kit (VMD151) and comprehensive multiplex solutions such as the RapidFor™ SARS-CoV-2 + FLU A/B + RSV + ADV + hMPV + HPIV Test Kit, Vitrosens enables healthcare providers and laboratories to strengthen their respiratory diagnostic strategies.
To request technical information, evaluation kits, or distribution opportunities for RapidFor™ respiratory diagnostics, contact sales@vitrosens.com and enhance your respiratory testing portfolio.
Key References
- Centers for Disease Control and Prevention. Human Parainfluenza Viruses Overview
- World Health Organization. Respiratory Virus Surveillance Guidelines
- Henrickson KJ. Parainfluenza viruses. Clinical Microbiology Reviews
- Weinberg GA et al. Epidemiology and clinical impact of human parainfluenza viruses. Journal of Infectious Diseases


