Premature rupture of membranes (PROM) remains a leading obstetric complication affecting approximately 8-10% of all pregnancies worldwide, with potentially serious consequences for both mother and baby. Early and accurate diagnosis of membrane rupture is essential to guide appropriate clinical management, prevent complications such as preterm birth and infection, and optimize maternal and neonatal outcomes. The RapidFor™ iGFBP-1 Rapid Test offers healthcare professionals a revolutionary point-of-care diagnostic solution, enabling swift, accurate detection of insulin-like growth factor-binding protein 1 (iGFBP-1) in vaginal secretions to confirm rupture of fetal membranes. This blog explores the clinical significance of PROM, the challenges of traditional diagnostic methods, and how the RapidFor™ iGFBP-1 test is transforming obstetric care.
Understanding Premature Rupture of Membranes (PROM)
Premature rupture of membranes refers to the breaking of the amniotic sac before the onset of labor, regardless of gestational age. When this occurs before 37 weeks of gestation, it is termed preterm premature rupture of membranes (PPROM), which accounts for approximately one-third of all preterm births. The amniotic membrane serves as a critical barrier protecting the fetus from infection while maintaining the optimal intrauterine environment necessary for fetal development.
PROM presents significant clinical challenges because delayed diagnosis can lead to serious maternal complications including chorioamnionitis (intrauterine infection), placental abruption, and postpartum hemorrhage. For the fetus and neonate, consequences may include preterm delivery, respiratory distress syndrome, neonatal sepsis, and in severe cases, increased perinatal mortality. The condition affects women across all demographics, though risk factors include previous PROM, intrauterine infection, tobacco use, and certain obstetric procedures.
The global burden of PROM is substantial, with studies indicating that PPROM complicates 2-4% of all pregnancies and is responsible for approximately 30-40% of preterm births worldwide. In low-resource settings where access to advanced obstetric care may be limited, accurate early detection becomes even more critical for appropriate triage and management decisions.
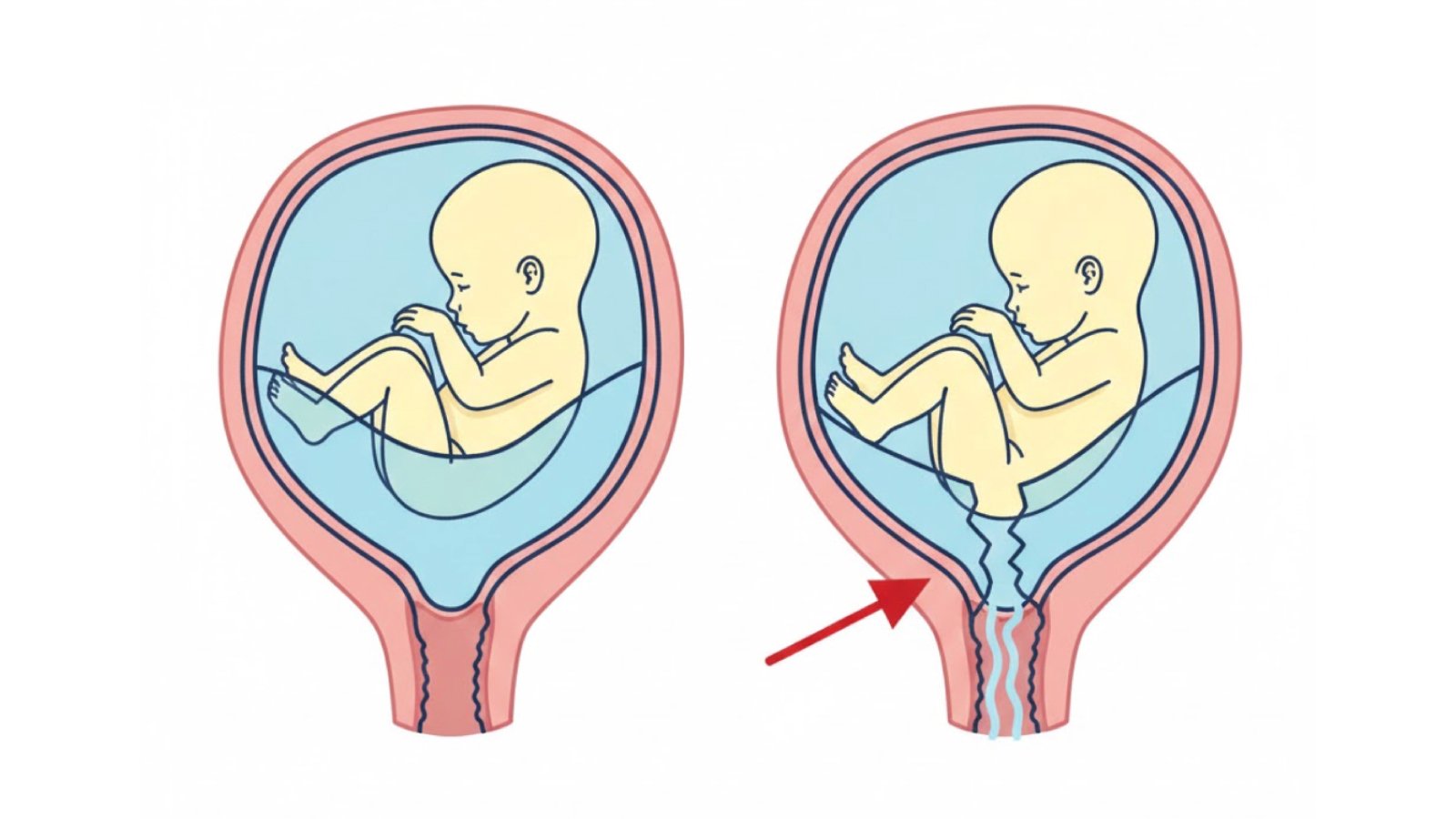
The Diagnostic Challenge: Why Accurate PROM Detection Matters
Accurately diagnosing rupture of membranes is not always straightforward, despite its clinical importance. While some cases present with obvious gushing of amniotic fluid, many women experience only subtle fluid leakage that can be confused with normal vaginal discharge, urinary incontinence (common in pregnancy), or excess cervical mucus. This diagnostic ambiguity creates a critical need for objective testing methods.
Traditional diagnostic approaches have included clinical history, sterile speculum examination to visualize amniotic fluid pooling, the ferning test (microscopic examination of dried vaginal fluid), and nitrazine pH testing. However, these methods have significant limitations. The ferning test can produce false-positive results from cervical mucus, semen, or fingerprints on the slide, while nitrazine testing may be affected by blood, semen, bacterial vaginosis, or alkaline antiseptics, leading to misdiagnosis in up to 30% of cases.
False-negative results are equally problematic, potentially delaying critical interventions and increasing infection risk. Conversely, false-positive diagnoses may lead to unnecessary hospitalization, inappropriate administration of corticosteroids or antibiotics, and potentially iatrogenic preterm delivery. The clinical consequences of misdiagnosis underscore the urgent need for more reliable, objective diagnostic tools.
Early and accurate detection of membrane rupture enables clinicians to implement timely interventions including corticosteroid administration for fetal lung maturation, antibiotic prophylaxis to prevent infection, and appropriate monitoring or delivery planning based on gestational age. These interventions have been shown to significantly improve neonatal outcomes and reduce healthcare costs associated with prolonged hospitalizations.
iGFBP-1: The Gold-Standard Biomarker for Membrane Rupture Detection
Insulin-like growth factor-binding protein 1 (iGFBP-1) has emerged as the most reliable biomarker for diagnosing rupture of fetal membranes. This protein is produced by the decidua (the modified endometrial lining during pregnancy) and is normally present in high concentrations in amniotic fluid but at very low levels in maternal blood and vaginal secretions when membranes are intact.
When membrane rupture occurs, amniotic fluid containing high concentrations of iGFBP-1 leaks into the vagina, creating a detectable signal that can be measured using immunochromatographic assays. The concentration of iGFBP-1 in amniotic fluid is approximately 100-1000 times higher than in maternal serum, providing an excellent diagnostic window. This substantial difference in concentration levels makes iGFBP-1 an ideal biomarker with high sensitivity and specificity.
Clinical studies have demonstrated that iGFBP-1 testing achieves sensitivity rates of 94-99% and specificity rates of 92-98% for detecting membrane rupture, significantly outperforming traditional diagnostic methods. Unlike pH-based tests, iGFBP-1 detection is not affected by common confounding factors such as blood, semen, urine, or vaginal infections, making it highly reliable across diverse clinical presentations.
The detection of iGFBP-1 provides an objective, quantifiable result that eliminates much of the subjectivity inherent in clinical examination and traditional testing methods. This objectivity is particularly valuable in borderline cases where clinical signs are ambiguous, enabling confident decision-making that directly impacts patient care pathways.
The RapidFor™ iGFBP-1 Rapid Test: Precision Diagnostics at the Point of Care
The RapidFor™ iGFBP-1 Rapid Test, developed by Vitrosens under the RapidFor™ brand, addresses the critical diagnostic gap in PROM detection by providing healthcare professionals with a fast, accurate, and easy-to-use point-of-care testing solution. This visually interpreted qualitative immunochromatographic test enables detection of iGFBP-1 in vaginal secretions within just 5 minutes, facilitating immediate clinical decision-making.
The test is specifically designed for professional use in obstetric settings, including labor and delivery units, emergency departments, and prenatal clinics. Its rapid turnaround time eliminates the delays associated with sending samples to external laboratories, enabling real-time assessment and immediate implementation of appropriate management protocols. This speed is particularly critical when managing patients presenting with suspected membrane rupture, where every minute counts in preventing ascending infection and other complications.
Key Features and Benefits:
- Exceptional Speed: Delivers reliable results in just 5 minutes, enabling immediate clinical decision-making and reducing patient anxiety during evaluation
- High Diagnostic Accuracy: Utilizes iGFBP-1 detection with sensitivity rates of 94-99% and specificity rates of 92-98%, minimizing false-positive and false-negative results
- Simple Operation: Requires minimal training, making it accessible to all healthcare professionals involved in obstetric care, from nurses to physicians
- Objective Results: Provides clear, visually interpreted results that eliminate subjectivity and reduce inter-observer variability
- No Special Equipment Required: Can be performed at the bedside without the need for microscopes, pH meters, or laboratory infrastructure
- Cost-Effectiveness: Reduces unnecessary hospitalizations and interventions associated with false-positive diagnoses while preventing complications from missed cases
- Point-of-Care Convenience: Enables testing in diverse settings including outpatient clinics, emergency departments, and remote healthcare facilities
The RapidFor™ iGFBP-1 test represents a significant advancement over traditional PROM diagnostic methods, combining the gold-standard biomarker with practical point-of-care testing technology that fits seamlessly into clinical workflows.
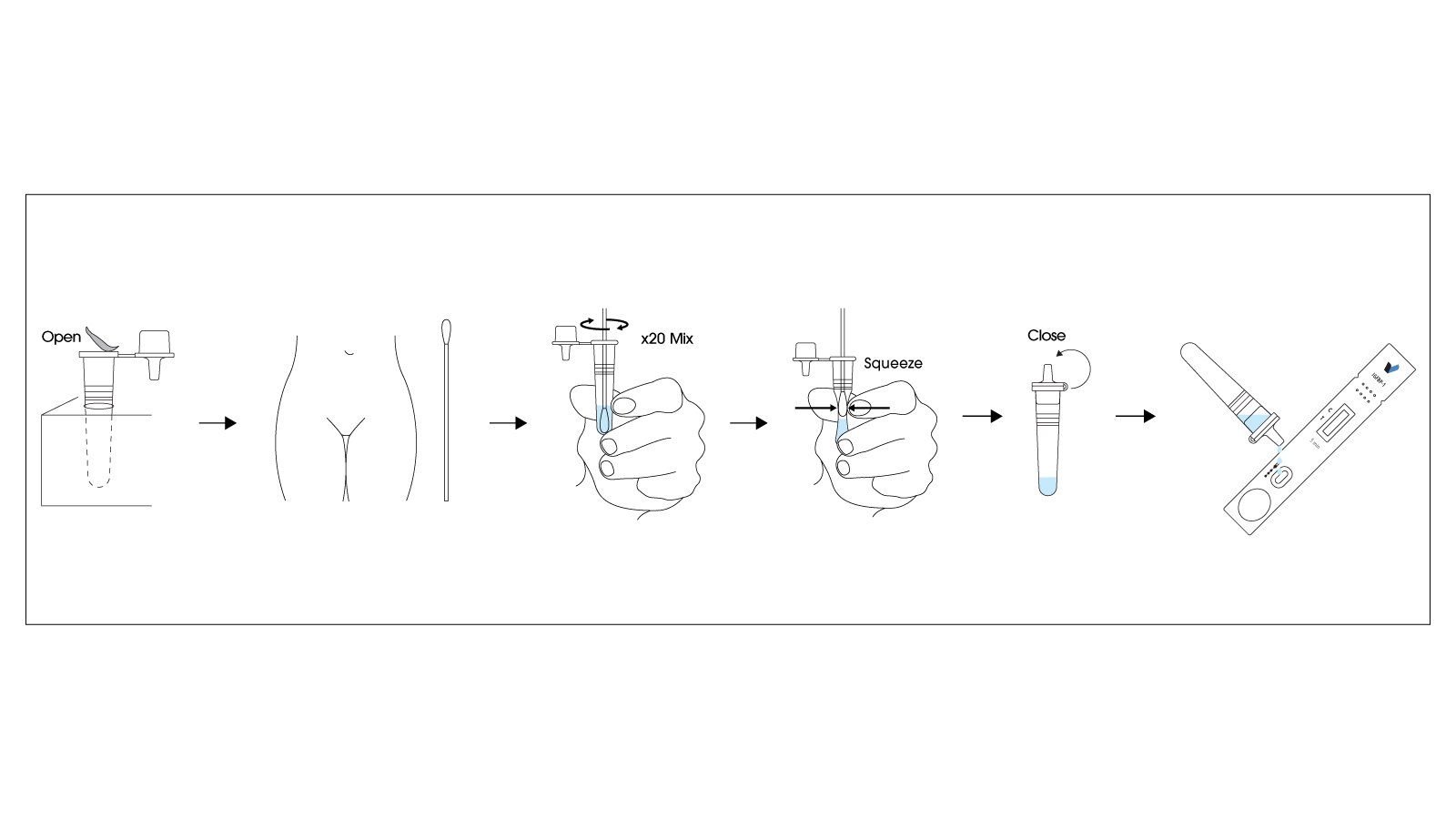
How to Use the RapidFor™ iGFBP-1 Rapid Test
Using the RapidFor™ iGFBP-1 Rapid Test is straightforward, requiring minimal training and no specialized laboratory equipment. The test can be performed quickly at the patient’s bedside, providing results within minutes:
- Patient Preparation: Position the patient in the lithotomy position for speculum examination. Ensure the vaginal area is clean; avoid using lubricants or antiseptics that might interfere with the test.
- Sample Collection: Using a sterile speculum examination, collect vaginal discharge from the posterior vaginal fornix using the provided sterile swab. Gentle rotation of the swab ensures adequate sample collection. This step should be performed carefully to avoid cervical contamination.
- Sample Application: Remove the test cassette from its protective pouch. Immediately insert the swab with collected vaginal secretions into the sample well of the test cassette, ensuring the swab makes contact with the absorbent pad.
- Incubation Period: Allow the test to develop for exactly 5 minutes at room temperature. Do not interpret results before 5 minutes have elapsed, as this may lead to inaccurate readings.
- Result Interpretation:
- Positive Result: Two colored lines appear—one in the control (C) region and one in the test (T) region. Any visible line in the test region, regardless of intensity, indicates a positive result suggesting rupture of membranes.
- Negative Result: Only one colored line appears in the control (C) region with no line in the test (T) region, suggesting intact membranes.
- Invalid Result: If no control line appears, the test is invalid and should be repeated with a new cassette.
Important Considerations: Results should be interpreted within 5-10 minutes of testing. Store test cassettes at 2-30°C and do not use beyond the expiration date. Always correlate test results with clinical findings, patient history, and other diagnostic information. If clinical suspicion remains high despite a negative result, consider repeat testing or alternative diagnostic methods.
Transforming Obstetric Care and Maternal Outcomes
The introduction of rapid, accurate iGFBP-1 testing has significant implications that extend far beyond individual patient diagnosis, fundamentally improving obstetric care delivery and maternal-fetal outcomes:
- Enhanced Clinical Decision-Making: Immediate, objective test results enable obstetricians to make confident, evidence-based decisions regarding admission, transfer, intervention timing, and delivery planning, reducing clinical uncertainty in ambiguous presentations.
- Improved Resource Allocation: Accurate diagnosis prevents unnecessary hospitalizations and interventions for false-positive cases while ensuring appropriate intensive monitoring for true-positive cases, optimizing healthcare resource utilization and reducing costs.
- Reduced Maternal and Neonatal Complications: Early detection of membrane rupture enables timely administration of corticosteroids for fetal lung maturation and antibiotic prophylaxis, significantly reducing rates of respiratory distress syndrome, neonatal sepsis, and maternal chorioamnionitis.
- Accessibility in Diverse Settings: The test’s simplicity and lack of equipment requirements make it suitable for implementation in resource-limited settings, rural clinics, and emergency departments where access to laboratory facilities may be limited, democratizing access to advanced diagnostic capabilities.
- Patient Satisfaction and Anxiety Reduction: Rapid, definitive answers reduce the anxiety and uncertainty patients experience when presenting with suspected membrane rupture, improving the overall care experience and patient-provider communication.
- Medicolegal Protection: Objective, documented test results provide clear evidence supporting clinical decision-making, protecting healthcare providers while ensuring patients receive appropriate standard-of-care management.
By integrating the RapidFor™ iGFBP-1 Rapid Test into obstetric protocols, healthcare facilities can establish evidence-based pathways that standardize PROM evaluation, reduce diagnostic variability, and ensure every patient receives timely, appropriate care regardless of provider experience level or clinical setting.
Conclusion
Accurate, timely diagnosis of premature rupture of membranes is essential for optimizing maternal and neonatal outcomes while preventing serious complications. The RapidFor™ iGFBP-1 Rapid Test represents a significant advancement in point-of-care obstetric diagnostics, combining the gold-standard iGFBP-1 biomarker with rapid, simple testing technology that can be implemented in virtually any clinical setting. With its exceptional accuracy, 5-minute turnaround time, and ease of use, this test empowers healthcare professionals to make confident, evidence-based decisions that directly improve patient care. As maternal healthcare continues to evolve toward more precise, accessible diagnostic solutions, the RapidFor™ iGFBP-1 test stands as a vital tool for protecting mothers and babies worldwide.
References
- American College of Obstetricians and Gynecologists (ACOG). “Premature Rupture of Membranes: ACOG Practice Bulletin, Number 217.” Obstetrics & Gynecology, 2020.
- Mercer, B.M. “Preterm Premature Rupture of the Membranes: Current Approaches to Evaluation and Management.” Obstetrics and Gynecology Clinics of North America, 2005.
- Igbinosa, I., Moore, F.A., & Johnson, C. “Premature Rupture of Membranes: Review of Diagnostic Methods.” American Journal of Perinatology, 2017.
- Rutanen, E.M., et al. “Insulin-like Growth Factor Binding Protein-1 as a Cervical Mucus Protein and a Marker of Ovarian Activity.” Fertility and Sterility, 1988.
- Cousins, L.M., et al. “Accuracy of a Rapid Bedside Test for Detection of Rupture of Membranes Using Insulin-like Growth Factor-binding Protein-1.” American Journal of Obstetrics & Gynecology, 2011.
- World Health Organization (WHO). “WHO Recommendations on Interventions to Improve Preterm Birth Outcomes,” 2015.
- Tita, A.T., & Andrews, W.W. “Diagnosis and Management of Clinical Chorioamnionitis.” Clinics in Perinatology, 2010.


