India faces a recurring public health challenge with Nipah virus outbreaks, particularly concentrated in Kerala State and most recently in West Bengal. With a staggering case fatality rate ranging from 40 to 75 percent and potential for human-to-human transmission, Nipah virus represents one of the most dangerous emerging infectious diseases in South Asia. The Vitrosens ChainFor™ Nipah Virus Real-Time PCR Detection Kit offers healthcare professionals a critical molecular diagnostic tool for rapid, accurate detection of this deadly pathogen. This blog explores the epidemiology of Nipah virus, the current outbreak situation in India, clinical challenges, and how advanced PCR technology is transforming outbreak response.
Nipah Virus: A High-Priority Pathogen with Pandemic Potential
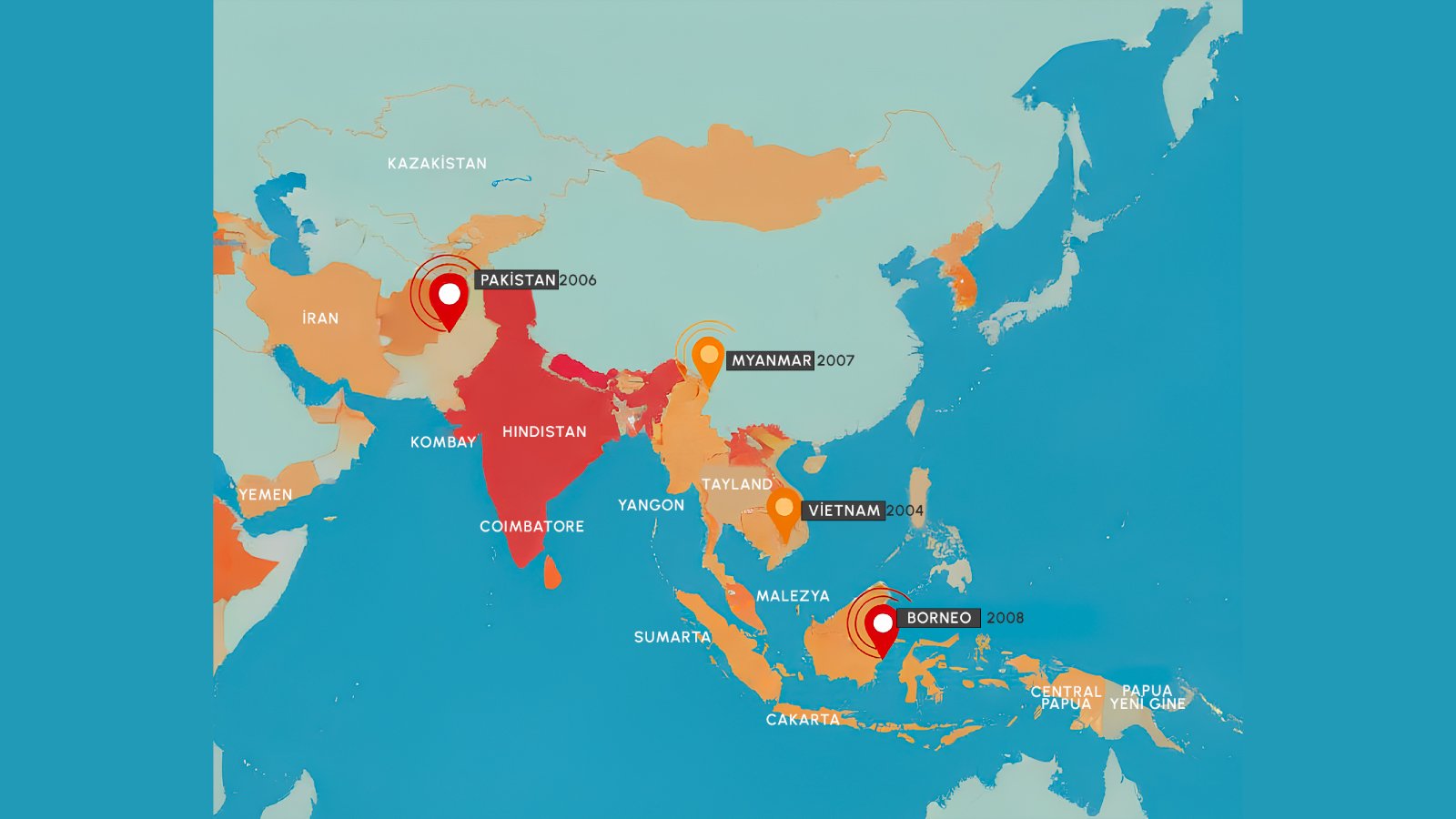
Nipah virus is a zoonotic paramyxovirus first identified in 1999 during an outbreak in Malaysia that affected pig farmers and resulted in 105 deaths among 265 cases. The virus belongs to the family Paramyxoviridae, genus Henipavirus, and derives its name from Sungai Nipah village in Malaysia where it was initially isolated. Fruit bats of the genus Pteropus serve as the natural reservoir for Nipah virus, harboring the pathogen without developing disease themselves.
The World Health Organization has classified Nipah virus as a priority pathogen due to its high mortality rate, potential for epidemic spread, and lack of approved vaccines or specific treatments. Clinical manifestations range from mild respiratory illness to severe acute encephalitis and acute respiratory distress syndrome. Between 40 and 75 percent of infected individuals succumb to the disease, with some outbreaks documenting case fatality rates approaching 100 percent. Survivors often face long-term neurological sequelae including memory loss, cognitive impairment, seizures, and personality changes.
India’s Recurring Nipah Crisis: Kerala and West Bengal Outbreaks
Since 2001, India has experienced multiple Nipah virus outbreaks with devastating consequences. The first Indian outbreak occurred in Siliguri, West Bengal, with 66 cases and a 74 percent mortality rate, notably demonstrating significant nosocomial transmission as 75 percent of patients were hospital staff or visitors. Kerala State has emerged as a particular hotspot, reporting nine separate outbreaks since 2018 with consistent recurrence patterns.
The 2024-2025 outbreak season has proven especially challenging. Kerala reported four confirmed cases between April and July 2025, including two deaths, marking the first-ever outbreak in Palakkad District. In January 2026, West Bengal confirmed five new cases near Kolkata, India’s third-most populous city, prompting urgent contact tracing and quarantine measures affecting nearly 100 individuals. These recurrent spillover events highlight ongoing risk associated with bat-human interface in these regions.
Healthcare workers face disproportionate risk during outbreaks. Among contacts placed under observation in the 2025 Kerala outbreak, 61 of 110 individuals in Palakkad and all 87 in Kozhikode were medical personnel, underscoring the critical importance of infection control measures in healthcare settings.
Transmission Pathways and Risk Factors
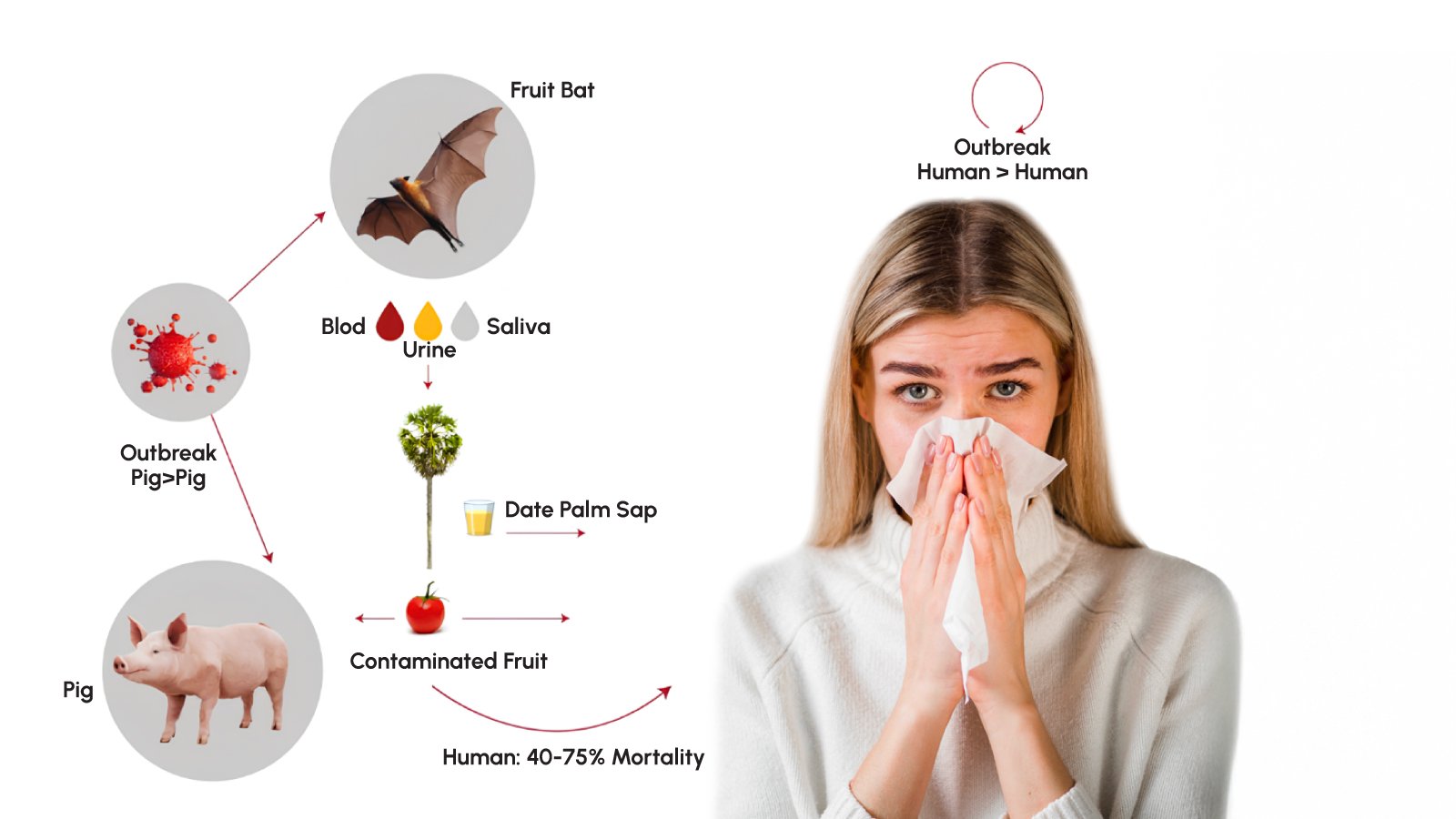
Understanding Nipah virus transmission is essential for effective outbreak prevention. The virus spreads through multiple pathways, creating complex epidemiological challenges. Primary transmission occurs through zoonotic spillover when humans consume raw date palm sap contaminated by bat urine or saliva, or eat fruits partially consumed by infected bats. In Bangladesh, approximately 21 of 23 index cases developed initial symptoms during the December through March date palm sap collection season.
Secondary transmission from animals represents another important pathway. Fruit bats drop partially eaten saliva-laden fruit that domestic animals consume, potentially becoming infected and subsequently transmitting the virus to humans through direct contact. The original Malaysian outbreak involved pig-to-human transmission in farm settings.
Human-to-human transmission poses the greatest concern for epidemic potential. The virus spreads through close contact with infected individuals’ secretions and excretions, particularly respiratory droplets from coughing patients. Research analyzing 248 Nipah cases in Bangladesh identified key risk factors for person-to-person transmission: male gender, age over 45 years, presence of respiratory symptoms, and severity of illness. Virtually all transmission events originated from patients with breathing problems who later died from their infections.
Healthcare settings present elevated transmission risk. The 2001 Siliguri outbreak demonstrated catastrophic nosocomial spread when an unidentified patient infected 11 others in hospital, who were then transferred to other facilities where subsequent transmission infected 25 staff and 8 visitors across four generations.
Clinical Presentation and Diagnostic Challenges
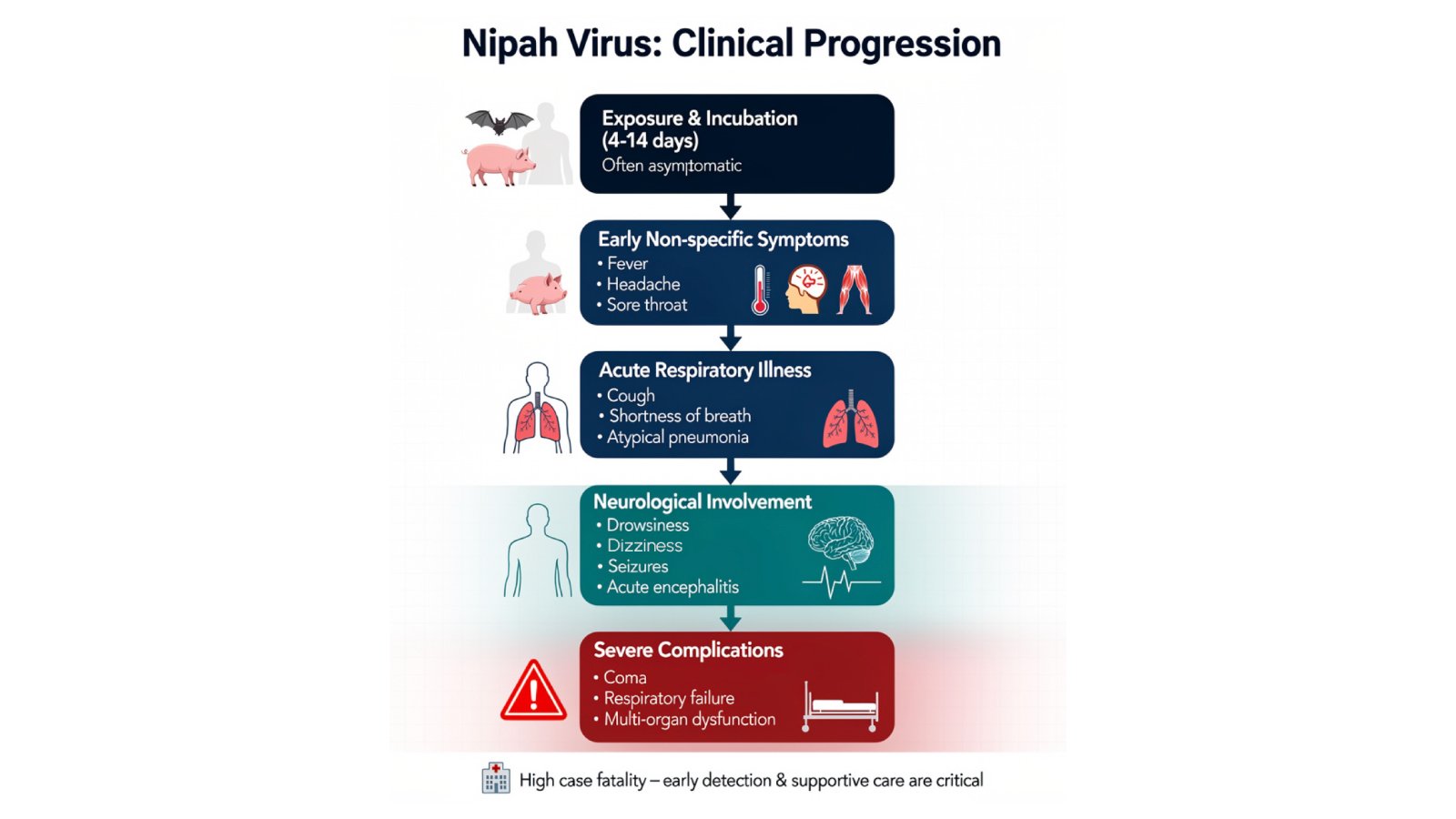
Nipah virus infection presents with nonspecific early symptoms that complicate timely diagnosis, a critical factor given the narrow window for effective intervention. The incubation period typically ranges from 4 to 14 days following exposure, though some cases have been reported months or even years later as dormant or latent infections.
Initial symptoms resemble common febrile illnesses: fever, headache, muscle pain, vomiting, and sore throat. This nonspecific presentation means diagnosis is often not suspected at time of presentation, hampering accurate identification and creating challenges for outbreak detection and infection control measures.
As infection progresses, patients develop more distinctive symptoms indicating central nervous system involvement. These include dizziness, drowsiness, altered consciousness, confusion, and neurological signs characteristic of acute encephalitis. Some patients develop atypical pneumonia with severe respiratory problems including acute respiratory distress syndrome. In severe cases, patients may experience seizures and enter coma within 24 to 48 hours of neurological symptom onset.
The variation in clinical manifestations between outbreaks adds diagnostic complexity. Kerala’s outbreak data indicate that acute encephalitis syndrome-predominant outbreaks in 2019, 2021, and 2024 showed no human-to-human transmission, while acute respiratory distress syndrome-predominant outbreaks in 2001, 2007, 2018, and 2023 demonstrated significant secondary spread. This variability suggests different viral strains or transmission dynamics requiring tailored diagnostic and containment approaches.
The Vitrosens ChainFor™ Nipah Virus Real-Time PCR Detection Kit: Precision Molecular Diagnostics
Early, accurate detection is the cornerstone of Nipah virus outbreak control. The Vitrosens ChainFor™ Nipah Virus Real-Time PCR Detection Kit represents a sophisticated molecular diagnostic solution specifically designed to address the urgent need for rapid pathogen identification in outbreak settings.
Key Features and Benefits:
- High Sensitivity and Specificity: The qPCR technology employed in the ChainFor™ Kit enables highly sensitive detection of Nipah virus genetic material, ensuring identification even at low viral loads during early infection stages when clinical symptoms may be nonspecific.
- Rapid Results: Real-time PCR provides results in significantly less time compared to traditional diagnostic methods, enabling prompt clinical decision-making and immediate implementation of infection control measures critical for containing outbreaks.
- Comprehensive Detection: The kit targets specific Nipah virus RNA sequences with primers and probes designed for maximum accuracy, minimizing false negative results that could lead to missed cases and continued transmission.
- Versatility: The kit is compatible with major Real-Time PCR platforms including Thermo Scientific Quant Studio, BioRad CFX systems, and other widely used thermal cyclers, facilitating rapid implementation in diverse laboratory environments.
How to Use the ChainFor™ Nipah Virus Real-Time PCR Detection Kit
Using the Vitrosens ChainFor™ Nipah Virus Real-Time PCR Detection Kit involves a systematic molecular diagnostic workflow designed for accurate pathogen detection. Laboratory personnel trained in PCR techniques and biosafety protocols should perform these procedures in certified biosafety level facilities.
- Sample Collection: Obtain clinical specimens from suspected Nipah virus-infected individuals following appropriate biosafety protocols. Suitable sample types include blood, serum, cerebrospinal fluid, throat swabs, nasal swabs, or respiratory secretions. Proper collection technique and immediate processing are essential for optimal test performance.
- Nucleic Acid Extraction: Use a viral RNA extraction kit to isolate and purify Nipah virus genetic material from clinical samples. The ChainFor™ system is optimized for use with Virasens Viral RNA Purification Kit, ensuring maximum recovery of viral nucleic acids while removing PCR inhibitors.
- Reaction Preparation: Prepare the PCR reaction mixture by combining the master mix, primers, and fluorescent probes specific to Nipah virus RNA sequences provided in the kit. Add the extracted nucleic acid to the reaction mix. Include positive and negative controls in each run to verify assay accuracy and identify potential contamination.
- PCR Amplification: Load prepared reactions into the thermal cycler and initiate the Real-Time PCR protocol according to kit specifications. The thermal cycler performs multiple cycles of heating and cooling to amplify target sequences while monitoring fluorescence data in real-time.
- Data Analysis and Result Interpretation:
- Analyze real-time PCR data by measuring cycle threshold values where fluorescence signal exceeds background levels
- Lower Ct values indicate higher viral load and stronger positive results
- Compare sample Ct values to positive and negative controls to validate results
- Positive results indicate presence of Nipah virus genetic material requiring immediate clinical action
- Negative results with appropriate controls suggest absence of detectable Nipah virus
Best practices include proper storage of kit components at recommended temperatures, adherence to biosafety protocols when handling potentially infectious materials, documentation of all procedures, and timely reporting of results to public health authorities for outbreak surveillance purposes.
Transforming Outbreak Response and Public Health Preparedness
The availability of rapid, accurate molecular diagnostic tools like the ChainFor™ Nipah PCR Kit fundamentally transforms outbreak response capabilities and strengthens public health preparedness in endemic regions.
- Enhanced Surveillance Systems: Real-time PCR testing enables systematic surveillance of acute encephalitis syndrome and acute respiratory distress syndrome cases, allowing early detection of Nipah spillover events before widespread transmission occurs. India has established a network of seventeen Virus Research and Diagnostic Laboratories equipped for such surveillance in West Bengal and Kerala.
- Rapid Outbreak Containment: Swift case identification through molecular diagnostics facilitates immediate implementation of containment measures including contact tracing, quarantine protocols, and infection control procedures that prevent secondary transmission. Data from Kerala outbreaks demonstrate that early detection combined with efficient containment prevented human-to-human spread in encephalitis-predominant outbreaks.
- Healthcare Worker Protection: Accurate diagnostic testing allows appropriate triage and isolation of confirmed cases, protecting healthcare workers who face disproportionate risk during outbreaks. Molecular confirmation enables rational deployment of personal protective equipment and infection prevention resources.
- Clinical Management Guidance: While no specific antiviral treatments are approved for Nipah virus, confirmed diagnosis guides supportive care approaches and allows consideration of experimental therapeutics under compassionate use protocols, potentially improving patient outcomes.
- Epidemiological Research: Molecular detection data contributes to understanding viral transmission dynamics, strain variations, and geographic distribution patterns essential for developing targeted prevention strategies and future vaccine candidates.
The integration of advanced molecular diagnostics into outbreak response protocols represents a critical component of India’s comprehensive One Health approach to Nipah virus management, combining human health surveillance, veterinary monitoring, and ecological assessment of bat reservoirs.
Conclusion
Nipah virus continues to pose a significant public health threat in India, with recurring outbreaks demonstrating the virus’s persistent presence and epidemic potential in the region. The high mortality rate, capacity for human-to-human transmission, and lack of specific treatments underscore the critical importance of rapid, accurate diagnostic capabilities. The Vitrosens ChainFor™ Nipah Virus Real-Time PCR Detection Kit provides healthcare professionals and public health laboratories with an essential molecular diagnostic tool that enables early case identification, prompt implementation of control measures, and enhanced outbreak surveillance. As India strengthens its preparedness for future Nipah events, advanced PCR technology will remain central to protecting healthcare workers, containing transmission, and ultimately saving lives in affected communities.
For more information about the ChainFor™ Nipah Virus Real-Time PCR Detection Kit, please contact us at sales@vitrosens.com.
References
- World Health Organization. Disease Outbreak News: Nipah Virus Infection – India. August 2025. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON577
- Centers for Disease Control and Prevention. Nipah Virus: Facts for Clinicians. Atlanta: CDC; 2024. Available from: https://www.cdc.gov/nipah-virus/hcp/clinical-overview/index.html
- Nikolay B, Salje H, Hossain MJ, et al. Transmission of Nipah Virus—14 Years of Investigations in Bangladesh. N Engl J Med. 2019;380(19):1804-1814.
- Kumar S, Salleh MZ, et al. Nipah virus outbreaks in India: A comprehensive update. Biomed Res Int. 2024;2024:4066641.
- Yadav PD, Sahay RR, et al. Encephalitis-predominant Nipah virus outbreaks in Kerala, India during 2024. J Infect Pub Health. 2025. doi: 10.1016/j.jiph.2025.102782
- The Lancet Regional Health – Southeast Asia. Improving clinical care of patients in Nipah outbreaks: moving beyond compassionate use. February 2025.
- Johns Hopkins Bloomberg School of Public Health. How Nipah Virus Spreads From Person to Person. Baltimore: Johns Hopkins; 2019.


