Antimicrobial resistance threatens to undermine decades of medical progress, yet rapid diagnostic tests offer healthcare professionals a powerful tool to combat this crisis. The integration of point-of-care diagnostics into primary care settings transforms how clinicians approach antibiotic prescribing, enabling evidence-based decisions that protect both individual patients and public health. This comprehensive exploration examines how rapid tests for C-reactive protein, procalcitonin, Group A Streptococcus, and influenza serve as guardians of appropriate antimicrobial use in frontline healthcare settings.
The Antimicrobial Resistance Crisis: Understanding the Scope
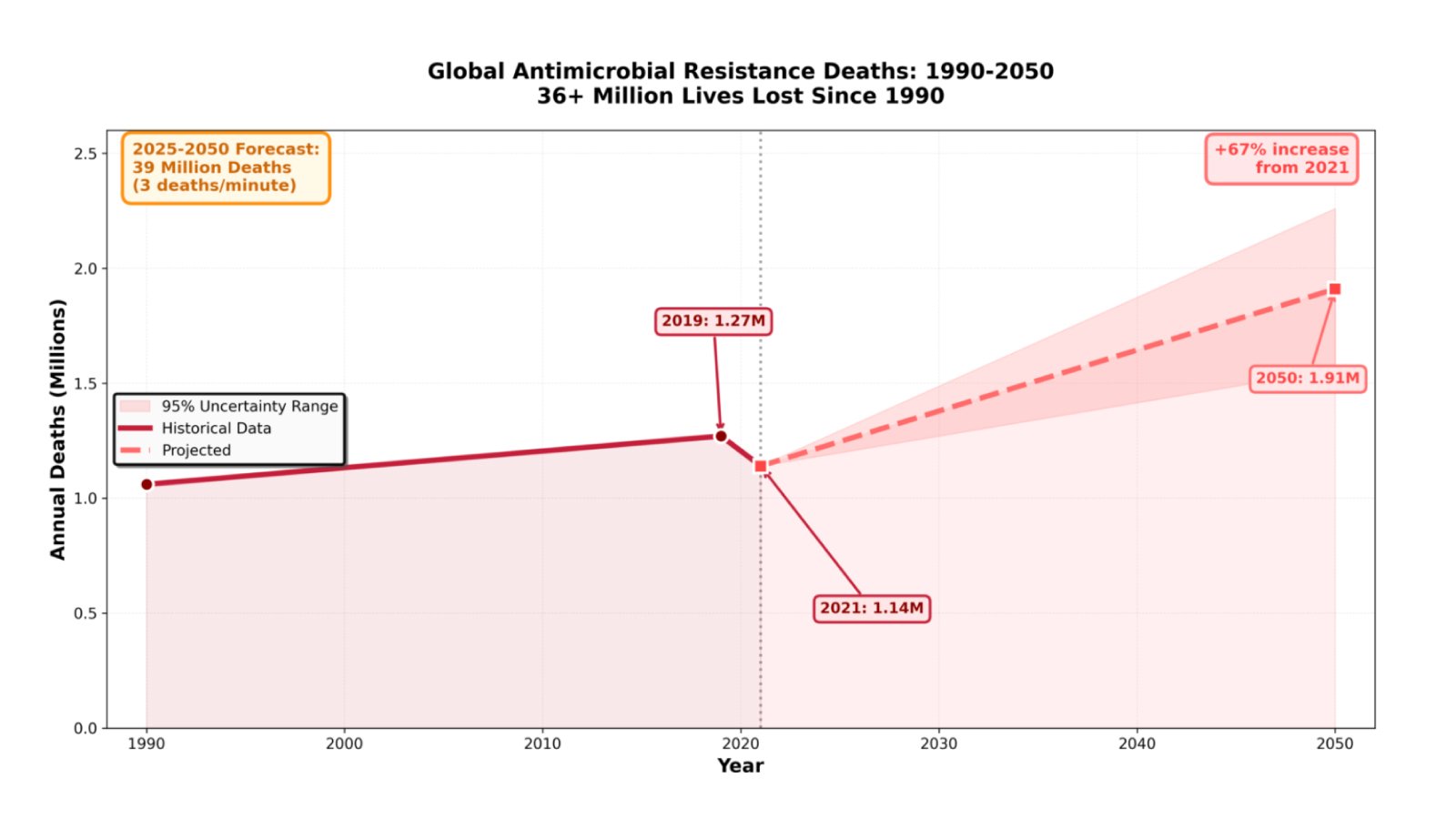
The global health community faces an escalating crisis that demands immediate action. According to the Global Research on Antimicrobial Resistance Project’s 2024 analysis, bacterial antimicrobial resistance has claimed over 36 million lives since 1990, with more than one million deaths occurring annually. Recent forecasts paint an even more concerning picture: between 2025 and 2050, AMR will directly cause 39 million deaths—equivalent to three deaths every minute.
The World Health Organization’s 2025 Global Antibiotic Resistance Surveillance Report reveals that resistance prevalence is highest in the WHO South-East Asian and Eastern Mediterranean Regions, where one in three reported infections demonstrates resistance to treatment. In the United States, more than 2.8 million antimicrobial-resistant infections occur annually, with six bacterial hospital-onset resistant infections increasing by 20% during the COVID-19 pandemic compared to pre-pandemic levels.
The economic burden compounds these human costs. Healthcare systems face estimated losses of $1-3.4 trillion per year by 2030, while treating infections caused by six antimicrobial-resistant pathogens costs more than $4.6 billion annually in the United States alone. Perhaps most troubling, the threat disproportionately affects older adults, with deaths in those aged 70 and older increasing by more than 80% between 1990 and 2021.
Primary Care’s Critical Role in Antibiotic Stewardship
Primary care clinics represent the frontline in the battle against antimicrobial resistance, accounting for the vast majority of antibiotic prescriptions. Respiratory tract infections alone trigger more than 12 million healthcare visits annually in the United States, yet studies consistently demonstrate that 60-80% of antibiotics prescribed for pharyngitis in primary care settings are unnecessary. This pattern extends across infection types, with research showing that inappropriate antibiotic prescriptions occur in approximately 30% of outpatient visits.
The challenge lies in diagnostic uncertainty. Viral and bacterial infections often present with overlapping symptoms, forcing clinicians to make treatment decisions without definitive microbiological confirmation. Traditional laboratory cultures require 24-48 hours for results, leaving providers dependent on clinical judgment alone during the initial consultation. Patient expectations further complicate decision-making, as many individuals arrive anticipating antibiotic prescriptions regardless of the actual infection etiology.
This diagnostic gap creates a perfect storm for antibiotic overuse. Clinicians, pressed for time and concerned about missing bacterial infections, frequently prescribe antibiotics as a precautionary measure. However, this approach contributes directly to resistance development while exposing patients to unnecessary side effects and costs.
C-Reactive Protein Testing: Reducing Diagnostic Uncertainty in Respiratory Infections
C-reactive protein point-of-care testing has emerged as one of the most evidence-based tools for antibiotic stewardship in primary care. This acute-phase protein, produced by the liver in response to inflammation, provides clinicians with objective data to distinguish between viral and bacterial infections during the initial patient consultation.
A comprehensive meta-analysis of 13 studies involving 9,844 participants demonstrated that CRP point-of-care testing significantly reduces immediate antibiotic prescribing for respiratory tract infections by 21% compared to usual care. The reduction proves even more substantial when CRP testing is combined with clear cut-off guidance, with studies showing up to 22% fewer antibiotic prescriptions in pediatric populations, particularly in low- and middle-income countries.
The clinical application follows evidence-based thresholds. For adults with lower respiratory tract infections, CRP values below 20 mg/L strongly suggest viral etiology, supporting watchful waiting without antibiotics. Values between 20-100 mg/L warrant careful clinical assessment and potential delayed prescribing, while levels exceeding 100 mg/L indicate probable bacterial infection requiring antibiotic treatment. In children, the threshold is even lower, with values below 20 mg/L supporting the avoidance of antibiotics when clinical assessment rules out severe infection.
The New England Journal of Medicine’s PACE trial demonstrated that CRP-guided antibiotic prescribing for COPD exacerbations reduced patient-reported antibiotic use from 77.4% to 57.0% while simultaneously improving COPD health status. Importantly, systematic reviews confirm that this reduction in prescribing does not compromise patient safety, with no significant differences in clinical recovery, symptom resolution, or hospital admissions between CRP-tested and standard care groups.
Cost-effectiveness analyses support widespread CRP implementation, showing that despite initial equipment costs, the reduction in unnecessary antibiotic use and associated complications creates net healthcare savings. However, broader adoption remains limited, with several national guidelines now recommending CRP point-of-care testing but implementation lagging in many primary care settings.
Procalcitonin: Precision Medicine for Sepsis and Severe Infections
Procalcitonin offers complementary diagnostic value to CRP, particularly in distinguishing bacterial from viral infections and guiding antibiotic duration. This peptide precursor of calcitonin rises more specifically in response to bacterial infections, with levels increasing within 2-4 hours of bacterial exposure and peaking at 12-24 hours.
The diagnostic advantage of procalcitonin lies in its superior specificity for bacterial infections compared to CRP. While CRP rises in response to any inflammatory stimulus, procalcitonin levels remain relatively stable during viral infections, autoimmune conditions, and non-infectious inflammation. This characteristic makes it particularly valuable in complex clinical scenarios where bacterial and viral infections might coexist.
Studies demonstrate that procalcitonin-guided algorithms routinely shorten antibiotic therapy duration by 1-2 days without increasing relapse risk. In emergency department settings, rapid procalcitonin testing combined with CRP measurement enables immediate risk stratification and more appropriate antibiotic initiation. Serial measurements track therapeutic response, allowing clinicians to confidently de-escalate or discontinue antibiotics when procalcitonin levels fall, typically indicating resolution of the bacterial infection.
The dual biomarker approach—combining CRP and procalcitonin measurement—provides the most comprehensive assessment. CRP offers high sensitivity for inflammation, while procalcitonin adds specificity for bacterial etiology. This combination supports both the decision to initiate antibiotics and the determination of appropriate treatment duration, addressing two critical antibiotic stewardship objectives simultaneously.
Group A Streptococcus Testing: Preventing Unnecessary Antibiotics for Pharyngitis
Streptococcus pyogenes accounts for 15-30% of pharyngitis cases in children and 5-15% in adults, yet studies indicate that 60-80% of adults with sore throat receive antibiotic prescriptions. Rapid antigen detection tests for Group A Streptococcus transform this pattern by providing definitive diagnosis during the initial consultation.
Modern rapid strep tests demonstrate sensitivity of 86-95% and specificity exceeding 95%, with results available in 5-10 minutes. This rapid turnaround enables immediate treatment decisions, eliminating the diagnostic uncertainty that drives precautionary prescribing. Current guidelines from the Infectious Diseases Society of America recommend RADT as the primary diagnostic modality, with backup throat culture required only for children under age 3 with negative results in settings where rheumatic fever risk is elevated.
The impact on antibiotic stewardship extends beyond simply identifying positive cases. Perhaps more importantly, negative rapid strep tests provide clinicians with confidence to withhold antibiotics, supported by patient education about viral pharyngitis natural history. Implementation studies demonstrate that when combined with clinical decision rules like the Centor or FeverPAIN scores, rapid strep testing reduces inappropriate antibiotic prescribing by 40-50% while maintaining appropriate treatment for confirmed bacterial infections.
The integration of rapid strep testing with standardized clinical protocols proves particularly effective. Healthcare systems implementing Centor screening tools combined with RADT report improvements in both documentation quality and prescribing appropriateness, with overall antibiotic prescription rates declining while ensuring all confirmed strep infections receive appropriate treatment.
A critical consideration involves distinguishing between colonization and active infection. Rapid tests detect streptococcal antigens but cannot differentiate between active infection and asymptomatic carriage, which occurs in up to 20% of the population during viral illnesses. This underscores the importance of appropriate patient selection based on clinical probability rather than reflexive testing of all sore throat presentations.
Influenza Testing: Optimizing Antiviral and Antibiotic Decisions
Rapid influenza diagnostic tests address a dual stewardship challenge: optimizing antiviral therapy while preventing unnecessary antibiotic prescriptions. Traditional multiplex PCR respiratory panels, while highly sensitive, require 12-48 hours for results. Rapid molecular tests reduce this time to 15-30 minutes, enabling real-time clinical decision-making.
The impact on antibiotic prescribing is substantial. Studies consistently demonstrate that positive rapid influenza tests reduce antibiotic prescription rates by 50-80% compared to clinical judgment alone. One emergency department study found antibiotic prescribing decreased from 39.4% to 8.9% after implementing rapid influenza PCR, while antiviral prescribing appropriately increased from 24.2% to 61.1%. The test turnaround time reduction from 27 hours to 3.5 hours proved critical, as it enabled prescribing decisions during the initial visit rather than requiring follow-up.
Rapid influenza testing also improves antiviral stewardship by identifying patients who would benefit from oseltamivir or other antivirals, particularly those at high risk for complications. The same emergency department study found that asthma, immunosuppression, and age under 5 years were significant predictors of appropriate antiviral prescribing when guided by rapid test results.
In primary care and urgent care settings, rapid influenza testing reduces unnecessary laboratory orders, imaging studies, and follow-up visits. Patients testing positive for influenza are less likely to receive chest X-rays, blood cultures, or additional diagnostic workup, streamlining care and reducing healthcare costs while maintaining clinical outcomes.
However, studies reveal an important caveat: the antibiotic-sparing effect of positive influenza tests appears more pronounced in pediatric than adult populations. This suggests that adult providers may be more likely to prescribe antibiotics for presumed secondary bacterial infections even with confirmed influenza, highlighting an opportunity for enhanced provider education and decision support.
The Vitrosens Solution: Comprehensive Rapid Diagnostic Tools for Antibiotic Stewardship
Vitrosens Biotechnology provides healthcare professionals with a comprehensive portfolio of rapid diagnostic solutions specifically designed to support antibiotic stewardship initiatives in primary care and point-of-care settings.
RapidFor™ hsCRP / CRP FIA Test Kit: Point-of-Care Inflammation Assessment
The Vitrosens hsCRP / CRP FIA Test Kit employs immunofluorescent technology to deliver quantitative C-reactive protein results in just 3 minutes. Designed for use with serum, plasma, or whole blood samples, this automated system eliminates subjective interpretation through objective numerical readouts. The kit’s Android-based interface streamlines workflow integration, while its ability to utilize fingerstick blood samples enhances patient convenience.
Key features include:
- Rapid Results: Objective readings in 3 minutes enable same-visit decision-making
- Quantitative Accuracy: Precise numerical values support evidence-based threshold application
- Sample Flexibility: Compatible with serum, plasma, and whole blood for maximum convenience
- User-Friendly Design: Minimal training required with automated reading and result printing
- Cost-Effectiveness: Reduces unnecessary antibiotic prescriptions and associated complications
The hsCRP/CRP Rapid Test Kit extends this capability by measuring both standard and high-sensitivity CRP in just 3 minutes, supporting cardiovascular risk assessment alongside infection diagnostics.

CRP/PCT Combo Test: Dual Biomarker Precision
For settings requiring maximum diagnostic precision, Vitrosens offers a combined CRP/PCT test delivering both biomarkers from a single cassette. This dual-biomarker approach is particularly valuable in emergency departments and urgent care settings where differentiating viral from bacterial infection with high confidence is critical.
The combination provides:
- Comprehensive Assessment: CRP sensitivity with PCT specificity in one test
- Enhanced Confidence: Dual markers reduce diagnostic uncertainty
- Faster Risk Stratification: Immediate bacterial versus viral differentiation
- Optimized Resource Use: Single test eliminates need for multiple platforms
- Sepsis Detection: Supports early recognition of severe bacterial infections
RapidFor™ Strep A Test Kit: Preventing Pharyngitis Overtreatment
The Vitrosens Strep A Rapid Test Kit utilizes lateral flow immunochromatography for qualitative detection of Group A Streptococcal antigens in oropharyngeal swab samples. Delivering rapid results, this test enables immediate treatment decisions for patients presenting with pharyngitis.
Distinguished by:
- Ultra-Fast Results: Fast turnaround time supports same-visit decisions
- High Accuracy: Sensitivity and specificity exceeding 95% for reliable diagnosis
- Simple Procedure: Straightforward swab collection and testing process
- Visual Clarity: Easy-to-read test lines eliminate interpretation errors
- Cost-Effective: Reduces unnecessary antibiotics while ensuring appropriate treatment
The less-invasive sampling and simple procedure make this test particularly suitable for pediatric populations and resource-limited settings where laboratory infrastructure may be unavailable.
Implementing Rapid Tests for Maximum Stewardship Impact
Successful antibiotic stewardship through rapid diagnostics requires more than simply introducing new technology. Healthcare organizations must implement comprehensive strategies that combine testing with clinical protocols, provider education, and patient communication.
Integration with Clinical Decision Rules
Rapid tests achieve maximum impact when combined with validated clinical decision tools. For respiratory infections, CRP testing should be integrated with clinical assessment and risk stratification. The approach includes documenting clinical findings, performing CRP testing when bacterial infection is possible but not certain, and applying evidence-based thresholds to guide antibiotic decisions. Similarly, Strep A testing benefits from integration with Centor or FeverPAIN scoring systems, testing only patients with intermediate probability scores rather than universal testing.
Provider Education and Decision Support
Healthcare professionals require training not just in test performance but in result interpretation and communication. Educational initiatives should emphasize understanding biomarker kinetics, recognizing appropriate indications for testing, interpreting results within clinical context, and explaining findings to patients effectively. Electronic medical record integration can support proper test ordering and documentation, embed clinical decision support algorithms, track prescribing patterns for quality improvement, and facilitate appropriate follow-up for pending results.
Patient Communication Strategies
Rapid test results provide a powerful tool for patient education, transforming potentially difficult conversations about antibiotic avoidance into teachable moments. When CRP values are low or influenza tests are positive, providers can use objective data to explain why antibiotics won’t help and may cause harm. Effective communication includes sharing the test result and its meaning, explaining the natural course of viral infections, discussing when to return if symptoms worsen, and providing explicit symptom management guidance.
Studies demonstrate that when providers use decision aids incorporating test results, patient satisfaction remains high (exceeding 90%) even when antibiotics are not prescribed. This suggests that patients value evidence-based care and objective diagnostic information over reflexive antibiotic prescribing.
Quality Monitoring and Feedback
Sustainable stewardship requires ongoing monitoring and provider feedback. Healthcare organizations should track antibiotic prescribing rates by condition and provider, assess rapid test utilization patterns and adherence to protocols, monitor patient outcomes including re-consultations and complications, and analyze cost-effectiveness including test costs versus antibiotic savings. Regular feedback to providers about their prescribing patterns, benchmarked against peers and evidence-based targets, reinforces appropriate antibiotic use while identifying opportunities for improvement.
Overcoming Barriers to Rapid Test Implementation
Despite strong evidence supporting rapid diagnostics for antibiotic stewardship, adoption remains suboptimal in many primary care settings. Several barriers impede widespread implementation, though each has viable solutions.
Cost considerations often dominate discussions, with concerns about equipment purchase, ongoing supply costs, and reimbursement uncertainty. However, economic analyses consistently demonstrate cost-effectiveness when accounting for avoided antibiotic prescriptions, reduced complications, and decreased follow-up visits. Many healthcare systems find that the return on investment becomes positive within 12-18 months, particularly in high-volume primary care practices.
Workflow integration challenges include concerns about testing adding time to already-compressed appointments and managing results that may not be immediately available. Solutions include optimizing test timing within the visit flow, utilizing medical assistants or nursing staff for test performance, and implementing point-of-care devices with minimal hands-on time. Many modern platforms provide automated reading and result printing, minimizing provider time investment.
Provider skepticism sometimes stems from concerns about test accuracy, particularly regarding false negatives and false positives. Comprehensive education about test performance characteristics, including sensitivity, specificity, and predictive values in relevant patient populations, helps build confidence. Emphasizing that tests support rather than replace clinical judgment addresses concerns about over-reliance on technology.
Reimbursement and regulatory considerations vary by region and healthcare system. In some countries, CRP point-of-care testing receives explicit reimbursement and guideline support, while others lag behind. Healthcare organizations can advocate for appropriate reimbursement policies while demonstrating value through quality metrics and cost savings data.
The Future of Rapid Diagnostics in Primary Care Stewardship
The landscape of rapid diagnostic testing continues to evolve, with several promising developments on the horizon that will further enhance antibiotic stewardship capabilities.
Multiplex molecular platforms capable of detecting multiple pathogens simultaneously are becoming more accessible for primary care settings. These tests can differentiate between bacterial and viral causes of respiratory infections, identify specific pathogens, and even detect resistance markers all within 15-30 minutes. While currently more expensive than traditional rapid tests, decreasing costs and increasing evidence of value promise broader adoption.
Artificial intelligence integration may enhance result interpretation and clinical decision support. Machine learning algorithms can incorporate rapid test results alongside clinical features, vital signs, and prior history to generate personalized treatment recommendations. These systems might identify patients at high risk for bacterial superinfection despite viral test positivity, or conversely recognize low-risk presentations where testing can be safely omitted.
Home-based and pharmacy-based testing represents another frontier. Patient-performed tests for conditions like strep throat or influenza could reduce healthcare visit burdens while maintaining appropriate diagnosis and treatment. Community pharmacies increasingly serve as antimicrobial stewardship touchpoints, with pharmacist-led testing and collaborative practice agreements enabling appropriate care outside traditional medical settings.
Novel biomarkers under investigation may provide even more precise bacterial versus viral discrimination. Host response biomarkers measuring the patient’s immune response rather than pathogen presence show promise for accurately identifying bacterial infections regardless of the specific causative organism. These tests could dramatically reduce diagnostic uncertainty in challenging presentations.
Conclusion: Rapid Tests as Guardians of Effective Antimicrobials
The antimicrobial resistance crisis demands decisive action across all healthcare settings, with primary care playing a pivotal role due to its predominance in antibiotic prescribing. Rapid diagnostic tests for C-reactive protein, procalcitonin, Group A Streptococcus, and influenza provide healthcare professionals with powerful tools to guide appropriate antibiotic use during initial patient encounters.
Evidence conclusively demonstrates that these tests reduce inappropriate antibiotic prescriptions by 20-50% across various clinical scenarios while maintaining or improving patient outcomes. The technology exists, the evidence base is robust, and the economic case is compelling. What remains is the imperative to implement these tools systematically and thoughtfully, supported by provider education, patient communication, and organizational commitment to antimicrobial stewardship.
Vitrosens Biotechnology’s comprehensive portfolio of rapid diagnostic solutions empowers primary care clinicians to practice evidence-based medicine at the point of care. By providing accurate, rapid results that inform clinical decision-making, these tests serve as guardians of antimicrobial effectiveness protecting both individual patients and future generations from the devastating consequences of resistance.
The path forward requires collaboration among healthcare providers, diagnostic manufacturers, payers, and policymakers to ensure that rapid diagnostic capabilities reach every primary care setting globally. Only through such comprehensive efforts can we preserve the life-saving power of antibiotics while combating the escalating threat of antimicrobial resistance.
For more information about implementing rapid diagnostic testing in your practice, contact Vitrosens Biotechnology at sales@vitrosens.com
References
- Murray CJL, et al. Global burden of bacterial antimicrobial resistance 1990-2021: a systematic analysis with forecasts to 2050. The Lancet 2024;404(10459):1199-1226.
- World Health Organization. Global Antibiotic Resistance Surveillance Report 2025. Geneva: WHO; 2025.
- Centers for Disease Control and Prevention. Antimicrobial Resistance: Facts and Statistics. Atlanta: CDC; 2025.
- Smedemark SA, et al. C-reactive protein point-of-care testing to reduce antibiotic prescribing for respiratory tract infections in primary care. Cochrane Database Syst Rev 2022.
- Van Hecke O, et al. Guidance on C-reactive protein point-of-care testing and complementary strategies to improve antibiotic prescribing for adults with lower respiratory tract infections in primary care. Front Med 2023;10:1166742.
- Butler CC, et al. C-Reactive Protein Testing to Guide Antibiotic Prescribing for COPD Exacerbations. N Engl J Med 2019;381:111-120.
- Cohen JF, et al. Group A Streptococcus pharyngitis in Children: New Perspectives on Rapid Diagnostic Testing and Antimicrobial Stewardship. J Pediatr Infect Dis Soc 2024;13(4):250-256.
- Centers for Disease Control and Prevention. Clinical Guidance for Group A Streptococcal Pharyngitis. Atlanta: CDC; 2025.
- Hansen GT, et al. Impact of rapid molecular diagnostics for influenza on antibiotic stewardship in the emergency department. J Antimicrob Chemother 2023.
- Infectious Diseases Society of America. Clinical Practice Guidelines for the Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza: 2018 Update. Clin Infect Dis 2019.


