WHO Alert: H5N1 Outbreaks Signal Need for Rapid, Targeted Testing
In July 2025, the World Health Organization issued a Disease Outbreak News report confirming multiple human cases of Influenza A (H5N1), including a fatal case linked to poultry exposure in Mexico. This growing pattern of zoonotic spillover underscores the urgent need for rapid, accurate, and subtype-specific diagnostic tools.
Since 2021, H5N1 clade 2.3.4.4b has spread across multiple continents in both domestic and wild birds. Clade 2.3.4.4b refers to a specific genetic lineage of the H5N1 avian influenza virus. It is known for its wide geographic spread and high pathogenicity in poultry and wild birds. As H5N1 continues to circulate in wild and domestic birds and occasionally jumps to humans early detection remains a cornerstone of effective containment and clinical management. Although human-to-human transmission remains rare, mutations that increase mammalian transmissibility have been observed.
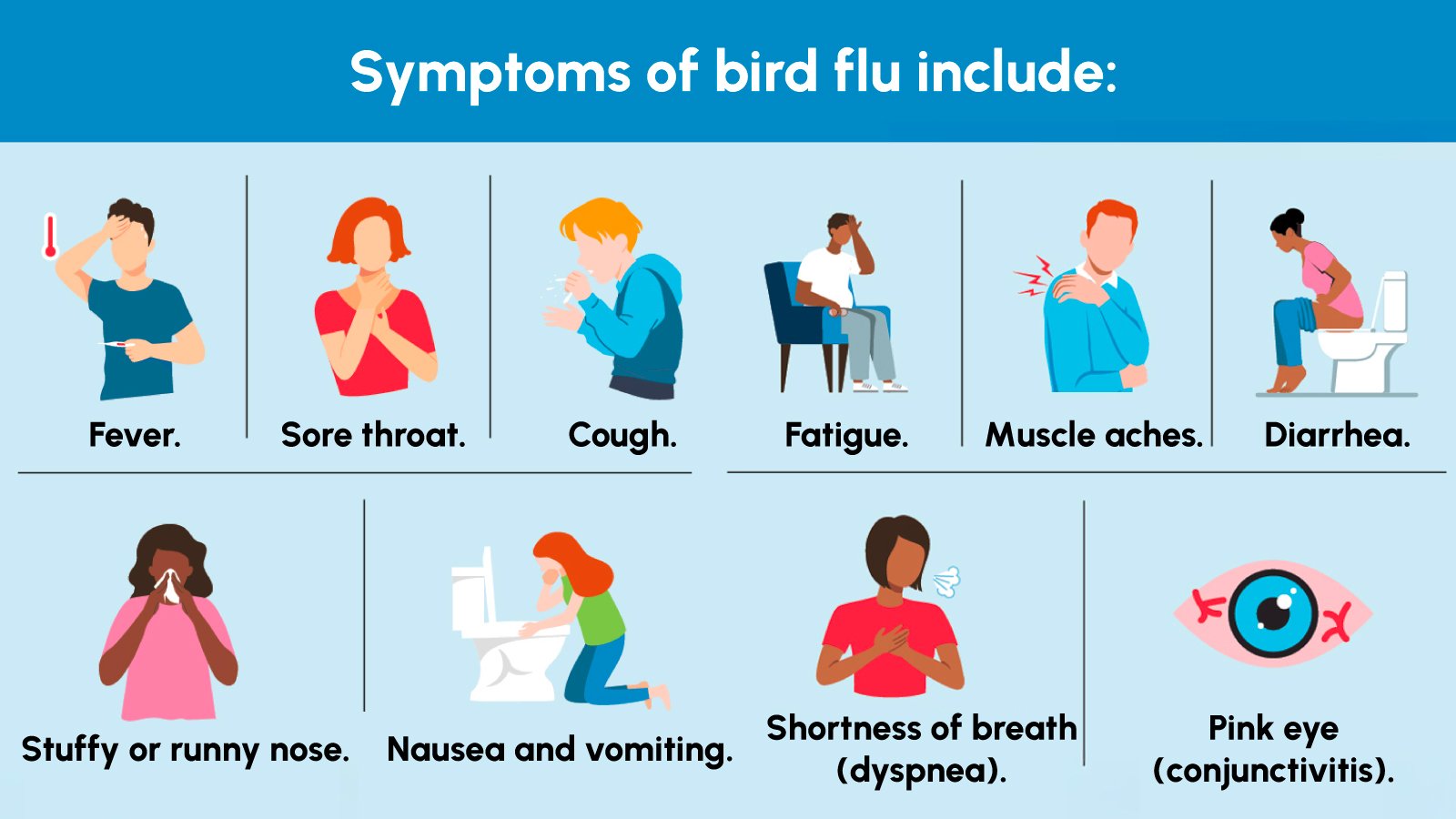
What is H5N1?
H5N1 is a highly pathogenic avian influenza (HPAI) virus that primarily infects birds but can occasionally cross the species barrier to humans. In humans, it often causes severe respiratory illness, with a reported mortality rate exceeding 50% in confirmed cases. The increasing number of H5N1 detections in mammals raises concern about potential adaptation to humans.
Transmission typically occurs through direct contact with infected birds or contaminated environments. Human-to-human transmission remains rare but is closely monitored by public health authorities due to pandemic potential.
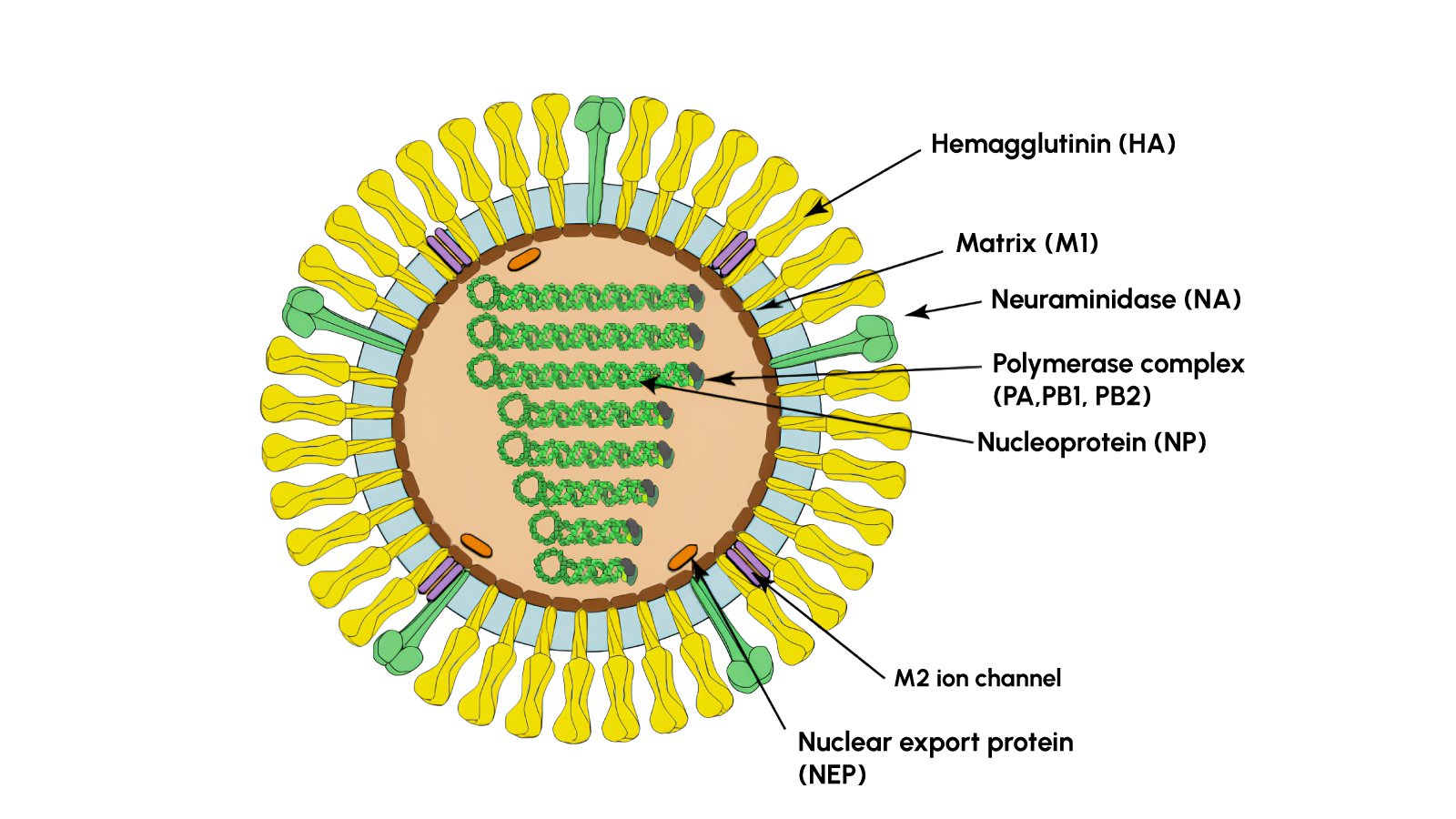
Introducing the Influenza A H5 Test Kit
In light of these risks, the RapidFor™ Influenza A H5 Test Kit has been developed to provide quick and specific identification of the H5 subtype of Influenza A virus, especially H5N1, using immunochromatographic technology (ICT), also known as lateral flow testing. It detects Avian-origin H5 influenza strains, including H5N1, H5N6, and H5N8 and Hemagglutinin subtype H5 antigens from clinical samples.
Why Rapid Subtype Detection Important
While broad influenza tests can indicate an Influenza A infection, only subtype-specific assays like this one can confirm the presence of the H5 hemagglutinin protein, which defines the avian-origin H5 subtype. This distinction is critical because:
- H5N1 carries higher mortality risk compared to seasonal flu
- Public health measures vary by subtype; H5 cases may trigger outbreak response protocols
- Antiviral treatment strategies may differ depending on the strain involved
Preparation Starts with Rapid Action
The RapidFor Influenza A H5 Test Kit enables clinicians and health authorities to quickly identify H5-positive cases, isolate them if needed, and report cases to surveillance networks. Its intuitive format makes it suitable for:
- Hospital emergency departments
- Public health field teams
- Border or airport screening units
- Veterinary-human interface investigation
Conclusion:
Exposure to infected birds or contaminated environments remains the most common source of human infection. As zoonotic influenza outbreaks continue to emerge, rapid, accurate testing tools are no longer optional they’re essential. The RapidFor™ Influenza A H5 Test Kit empowers professionals to detect high-risk H5 strains at the point of need, supporting faster decisions and better outcomes.
Protecting communities from avian influenza starts with rapid, subtype-specific detection.
For product information, evaluation kits, or technical consultation, contact the Vitrosens team today at sales@vitrosens.com
Key Reference:
- World Health Organization (5 July 2025). Disease Outbreak News; Avian Influenza A (H5N1) in Cambodia Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON575
- Vitrosens Biotechnology. Influenza A H5 Rapid Test Kit – Technical Manual. 2025.
- U.S. Centers for Disease Control and Prevention (CDC). Wastewater Data for Avian Influenza A(H5). https://www.cdc.gov/nwss/rv/wwd-h5.html


