Legionnaires’ disease is a serious and potentially life-threatening illness that often goes undetected in its early stages due to nonspecific symptoms. Caused by the bacterium Legionella pneumophila, this respiratory infection is responsible for thousands of hospitalizations and fatalities each year. The good news? With modern rapid diagnostic technology, early detection is more accessible now.
The RapidFor Legionella pneumophila Rapid Test Kit offers an accurate, user-friendly solution to help healthcare providers identify infections quickly with improving treatment outcomes and preventing complications. In this blog, we’ll break down what Legionella is, why diagnosis matters, and how this kit offers a smarter way forward in respiratory diagnostics.

What is Legionella pneumophila?
Legionella pneumophila is a species of bacteria found in freshwater environments, such as lakes and streams. However, it becomes a threat to human health when it grows and spreads in man-made water systems like:
- Air conditioning systems
- Hot tubs
- Showers
- Plumbing systems
People become infected by inhaling tiny droplets of water that contain the bacteria. Once inhaled, the bacteria travel to the lungs and cause Legionnaires’ disease, a type of pneumonia, that can range from mild respiratory symptoms to severe, life-threatening illness.
Symptoms of Legionnaires’ Disease
The disease typically develops 2 to 10 days after exposure and presents with symptoms such as:
- High fever and chills
- Cough (often dry or slightly productive)
- Muscle aches
- Shortness of breath
- Headache
- Fatigue
- Gastrointestinal symptoms like diarrhea or nausea
Because the early symptoms are similar to those of the flu or other forms of pneumonia, Legionnaires’ disease is often misdiagnosed or diagnosed too late. In severe cases, it can cause respiratory failure, kidney damage, or even death. It can cause these conditions especially in individuals with weakened immune systems, smokers, the elderly, or patients with chronic lung conditions.
The Importance of Early and Accurate Diagnosis
Legionnaires’ disease affects an estimated 25,000 to 100,000 people annually in the United States, with a mortality rate ranging from 25% to 40% if not treated early. Early diagnosis followed by prompt antibiotic therapy can dramatically reduce complications and fatalities.
Common Risk Groups Include:
- Hospitalized or elderly patients
- People with COPD or asthma
- Smokers or individuals with alcohol dependency
- Immunocompromised patients (e.g., cancer, transplant recipients)
Traditional diagnostic methods for Legionella, such as culture from sputum or lung tissue, are time-consuming and often inconclusive. Serological testing requires paired samples taken weeks apart. That’s why rapid, urine-based testing has become a key strategy for diagnosing Legionella pneumophila, especially serogroup 1, which causes over 70% of Legionella infections.
Introducing the RapidFor Legionella pneumophila Rapid Test Kit
The RapidFor Test Kit for Legionella pneumophila is a qualitative chromatographic immunoassay designed to detect the presence of Legionella antigen in human urine samples. The test specifically identifies L. pneumophila serogroup 1, responsible for the vast majority of Legionnaires’ disease cases.
Key Advantages
- Urine-Based Testing
Non-invasive and easy to collect, especially from hospitalized or elderly patients. - Rapid Results in 10 Minutes
Allows clinicians to make fast decisions in critical care settings. - Simple and Safe to Use
No need for complex lab equipment. Ideal for hospitals, clinics, and even mobile healthcare units. - Room Temperature Storage
Stable between 2°C and 30°C and no cold chain needed.
How to Use the RapidFor Legionella Rapid Test Kit
Kit Components (25 Tests/Box):
- 25 test cassettes (individually pouched)
- 25 single-use droppers
- 1 instruction for use (IFU)
Test Procedure:
- Sample Collection
- Use a fresh urine sample or store refrigerated at 2–8°C for up to 48 hours.
- Frozen samples should be thawed and mixed well before use.
- Prepare the Test
- Bring the cassette and urine sample to room temperature (15–30°C).
- Open the foil pouch only when ready to test.
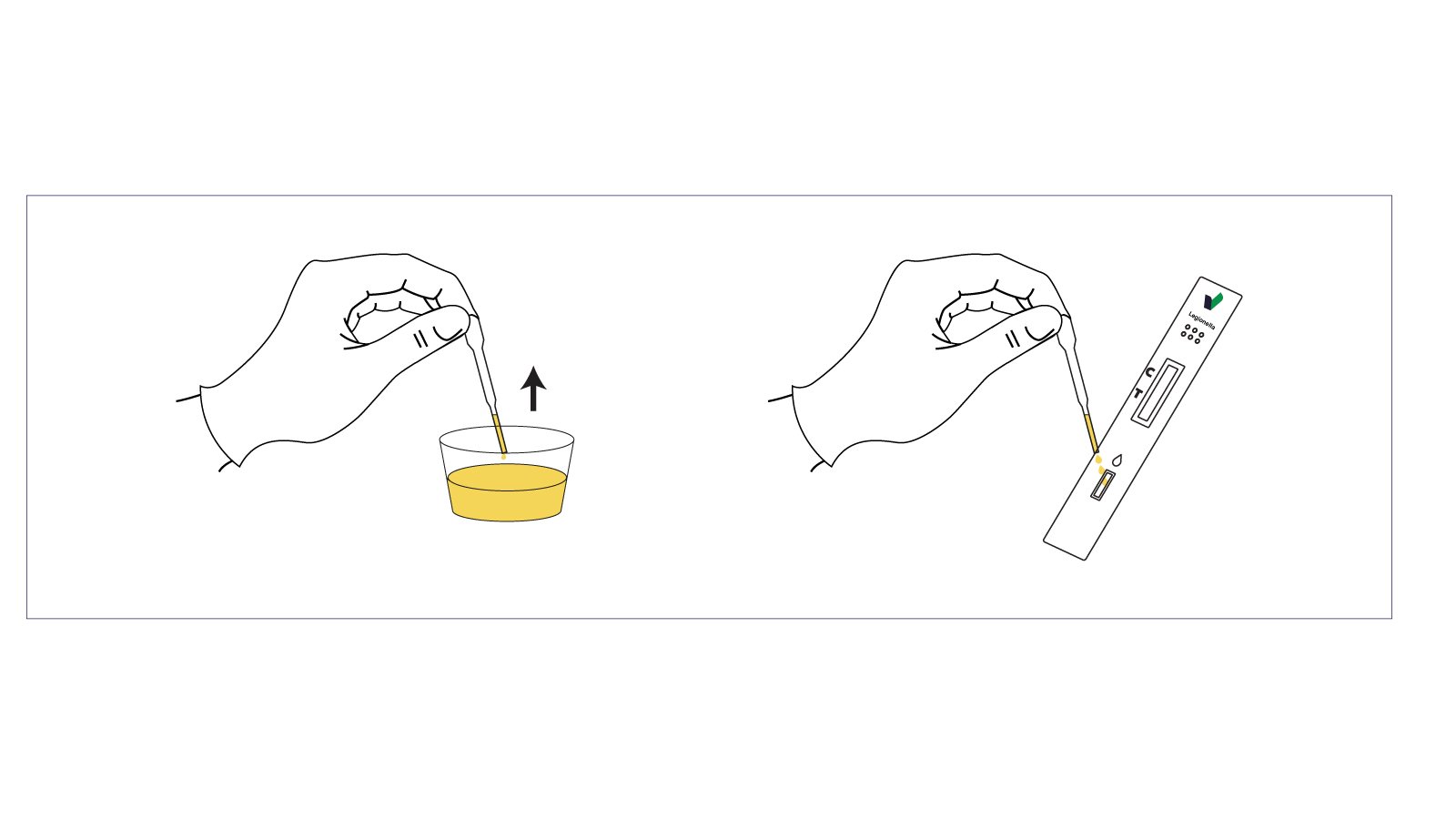
- Apply the Sample
- Use the provided dropper to place 3 drops of urine into the sample well (marked “S”).
- Wait and Read
- Results appear within 10 minutes.
- Do not read results after 15 minutes, as this may cause false interpretations.
Result Interpretation:
Test Line (T) |
Control Line (C) |
Interpretation |
Visible |
Visible |
Positive for Legionella |
Absent |
Visible |
Negative |
Absent or unclear |
Absent |
Invalid, repeat test |
Why Choose the RapidFor Legionella Test Kit?
- Faster Than Culture or PCR
Culture methods can take days. This test gives you actionable results in just 10 minutes. - Ideal for Emergency or ICU Use
Time-sensitive settings benefit from instant insights and faster decision-making. - Supports Outbreak Control
In suspected nosocomial or environmental outbreaks, rapid testing helps isolate and treat patients early. - Cost-Effective
No need for expensive instrumentation or specialized training with just a dropper, a cassette, and 10 minutes.
Storage and Handling Tips
- Store test kits in original packaging at 2–30°C with 40–60% humidity
- Use test cassettes within one hour of opening pouch
- Do not reuse any components
- Dispose of used materials according to local biohazard protocols
Conclusion: Diagnose with Confidence, Act with Speed
Legionnaires’ disease can go from mild to deadly in a matter of days. Fast, accurate diagnosis is not a luxury. It’s a clinical necessity. The RapidFor Legionella Pneumonia Rapid Test Kit empowers healthcare professionals with a trusted, easy-to-use tool for early detection, timely treatment, and better patient outcomes.
Whether you’re managing a respiratory ward, a long-term care facility, or a field hospital, this test belongs in your frontline diagnostic toolkit.
References
- CDC – Legionella (Legionnaires’ Disease and Pontiac Fever).
https://www.cdc.gov/legionella/index.html - Yu VL, et al. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired Legionellosis. Clin Infect Dis. 2002.
- European Centre for Disease Prevention and Control (ECDC). Surveillance report on Legionnaires’ disease. 2023.


